��Ŀ����
����Ŀ���Ͼɹ��̽������к��н���Ag(�������������Բ���)���ӹ�������ȡ���� Ag �Ĺ����������¡���ش��������⡣

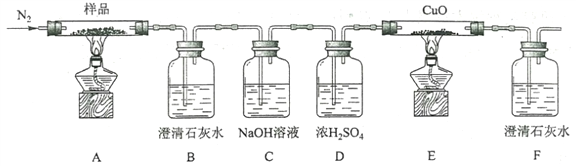
(1)�������������� 80 �� �����½��У�ʹ�õļ��ȷ�ʽΪ______________________��
(2)NaClO��Һ��Ag ��Ӧ�IJ���ΪAgCl��NaOH ��O2���÷�Ӧ�Ļ�ѧ����ʽΪ____________�����������HNO3���� NaClO����Ag���ӷ�Ӧ����ĽǶȷ�������ȱ����______________��
(3)�����ˢ���ϴ���������ʵ�����Ϊ_____________________________________��
(4)����10���İ�ˮ�ܽ�AgCl���壬 AgCl�� NH3H2O�� 1:2 ��Ӧ������ Cl����һ��������_____(�������ӵĻ�ѧʽ)����Һ��ʵ�ʷ�Ӧ�У���ʹ��ˮ����������������Ҳ��������AgCl���壬���ܵ�ԭ����__________________________________________��
(5)���������� 0.1 mol N2H4H2O�ɡ���ԭ���õ�_____ g Ag�ĵ��ʡ�
���𰸡�ˮԡ���� 4Ag+4NaClO+2H2O=4AgCl+4NaOH+O2�� ���ɵ��������Ⱦ���� ��©���м�������ˮ��������ȫ��û��ʹϴ��Һ��Ȼ���£��ظ�2~3�� ��Ag��NH3��2���� ��ˮ�ܽ�AgCl�ķ�Ӧ�ǿ��淴Ӧ�����ܽ��е��� 43.2
��������
(1)��ȷ�����¶ȣ���Ҫˮԡ���ȣ�
(2)���ݵ�ʧ�����غ���ƽNaClO��Һ��Ag ��Ӧ�ķ���ʽ��
(3)����ʵ������淶�ش�ϴ��������IJ���������
(4) ����ԭ���غ��ж�AgCl�� NH3H2O�� 1:2 ��Ӧ���ɵ������ӣ�
(5)���ݵ�ʧ�����غ��������Ag��������
(1)�������������� 80 �� �����½��У�ʹ��ˮԡ���ȣ�
(2)���ݵ�ʧ�����غ㣬NaClO��Һ��Ag ��Ӧ����AgCl��NaOH ��O2�ķ���ʽ��4Ag+4NaClO+2H2O=4AgCl+4NaOH+O2������������������������ԭ�����ǵ����������Ⱦ������
(3)�����ˢ���ϴ���������ʵ�����Ϊ��©���м�������ˮ��������ȫ��û��ʹϴ��Һ��Ȼ���£��ظ�2~3��
(4) AgCl�� NH3H2O�� 1:2 ��Ӧ����ʽ��AgCl+2NH3H2O![]() ��Ag��NH3��2��Cl���������ɵ��������ǣ�Ag��NH3��2���������ڿ��淴Ӧ���ܽ��е��ף����Լ�ʹ��ˮ����������������Ҳ��������AgCl���壻
��Ag��NH3��2��Cl���������ɵ��������ǣ�Ag��NH3��2���������ڿ��淴Ӧ���ܽ��е��ף����Լ�ʹ��ˮ����������������Ҳ��������AgCl���壻
(5) N2H4H2O���Ag��NH3��2��Cl ����������ԭ��Ӧ�����������ǵ�������ԭ������Ag��0.1 mol N2H4H2O��Ӧת��0.4mol���ӣ����ݵ�ʧ�����غ㣬�ɡ���ԭ���õ�0.4mol Ag������Ϊ0.4mol��108g/mol= 43.2g��