��Ŀ����
18��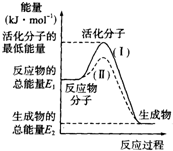 ��1���ڻ�ѧ��Ӧ�У�ֻ�м�����������ƽ�������ߵö�ķ�Ӧ����ӷ�����ײʱ�ſ��ܷ�����ѧ��Ӧ����Щ���ӱ���Ϊ����ӣ�ʹ��ͨ���ӱ�ɻ��������Ҫ�ṩ������ȵ������л�ܣ��䵥λͨ����kJ•mol-1��ʾ��������۲���ͼ��Ȼ��ش����⣮
��1���ڻ�ѧ��Ӧ�У�ֻ�м�����������ƽ�������ߵö�ķ�Ӧ����ӷ�����ײʱ�ſ��ܷ�����ѧ��Ӧ����Щ���ӱ���Ϊ����ӣ�ʹ��ͨ���ӱ�ɻ��������Ҫ�ṩ������ȵ������л�ܣ��䵥λͨ����kJ•mol-1��ʾ��������۲���ͼ��Ȼ��ش����⣮ͼ����ʾ��Ӧ�Ƿ��ȣ�����ȡ����ȡ�����Ӧ���÷�Ӧ��Ҫ�����Ҫ������Ҫ�������ȣ��÷�Ӧ�ġ�H=E2-E1 ���ú�E1��E2�Ĵ���ʽ��ʾ����
��2��1.00L1.00mol•L-1 H2SO4��Һ��2.00L1.00mol•L-1 NaOH��Һ��ȫ��Ӧ���ų�114.6kJ��������ʾ���к��ȵ��Ȼ�ѧ����ʽΪNaOH��aq��+$\frac{1}{2}$H2SO4��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ/mol��
��3����֪16g��������ȫȼ��ʱ�ų�148.4kJ���������÷�Ӧ���Ȼ�ѧ����ʽ��S��s��+O2��g��=SO2��g������H=-296.8 kJ/mol��
��4�����亽��������������N2H4��Ϊȼ�ϣ�N02Ϊ�ƽ�����ȼ�����ɵ�����ˮ�����������¶ȿɴ�2700�棬��֪��N2��g��+2O2��g���T2N02��g����H=+67.7kJ-mol-1��
N2H4��g��+02��g���TN2��g��+2H20��g����H=-534KJ-mol-1��
��N2H4��NO2��Ӧ��˵������ȷ���ǣ�������
A��N2H4��NO2��ӦʱNO2��������
B��������������������ǻ�ԭ����
C������1molN2ת��8mol����
D���÷�Ӧ���Ȼ�ѧ����ʽΪ2N2H4��g��+2NO2��g���T3N2��g��+4H20��g����
���� ��1������ͼ�������Ӧ��������������������������Ӧ���ȣ��ڻ�ѧ��Ӧ�У�ֻ�м�����������ƽ�������ߵö�ķ�Ӧ����ӷ�����ײʱ���ܷ�����ѧ��Ӧ��������Ҫ��������Ӧ���ʱ�=�����������-��Ӧ���������
��2���к��ȵĸ��ϡ��ǿ���ǿ�Ӧ����1molˮ���ų�����������к��ȣ��Դ���д�к��ȵ��Ȼ�ѧ����ʽ
��3�������Ȼ�ѧ����ʽ����д������֪����ѧ�������뷴Ӧ�ȳ����ȣ���ע��������ʵľۼ�״̬��
��4�����ݸ�˹���ɷ������
��� �⣺��1������ͼ�������Ӧ��������������������������Ӧ���ȣ��ڻ�ѧ��Ӧ�У�ֻ�м�����������ƽ�������ߵö�ķ�Ӧ����ӷ�����ײʱ���ܷ�����ѧ��Ӧ��������Ҫ��������Ӧ���ʱ�=�����������-��Ӧ��������TE2-E1��
�ʴ�Ϊ�����ȣ���Ҫ��E2-E1 ��
��2��1.00L 1.00mol/L H2SO4��Һ��2.00L 1.00mol/L NaOH��Һ��ȫ��Ӧ���ų�114.6kJ��������������2molˮ�ų�114.6kJ����������Ӧ�ķ�Ӧ��Ϊ-11.46kJ/mol���к���Ϊ-57.3kJ/mol�����к��ȵ��Ȼ�ѧ����ʽ��NaOH��aq��+$\frac{1}{2}$H2SO4��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ/mol��
�ʴ�Ϊ��NaOH��aq��+$\frac{1}{2}$H2SO4��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ/mol��
��3��16g��������ȫȼ��ʱ�ų�148.4kJ����������1molS��ȫȼ��ʱ�ų��ų�296.8kj���������Ȼ�ѧ����ʽΪ��S��s��+O2��g��=SO2��g������H=-296.8 kJ/mol��
�ʴ�Ϊ��S��s��+O2��g��=SO2��g������H=-296.8 kJ/mol��
��4����֪��N2��g��+2O2��g���T2NO2��g����H=+67.7kJ/mol ��
N2H4��g��+O2��g���TN2��g��+2H2O��g����H=-534kJ/mol ��
�٢�������2����-�ٵõ�2N2H4��g��+2NO2��g���T3N2��g��+4H2O��g����H=2����-��=2����-534kJ/mol��-��+67.7kJ/mol��=-1135.7kJ/mol��
A����Ӧ��NO2����N2��NԪ�ػ��ϼ۽��ͣ�����ԭ��Ϊ����������A��ȷ��
B����Ӧ�ķ���ʽΪ2N2H4��g��+2NO2��g���T3N2��g��+4H2O��g����ֻ��NԪ�ػ��ϼ۷����仯���������������������ǻ�ԭ�����B��ȷ��
C���ɷ���ʽ��֪2molNO2�μӷ�Ӧ������3molN2��ת�Ƶ������ʵ���Ϊ2��4mol=8mol��������1molN2��ת��$\frac{8}{3}$mol���ӣ���C����
D�������Ϸ�����֪�÷�Ӧ���Ȼ�ѧ����ʽ��2N2H4��g��+2NO2��g���T3N2��g��+4H2O��g����H=-1135.7 kJ•mol-1����D����
�ʴ�Ϊ��CD��
���� �����ۺϿ����к��ȡ���˹�����Լ��Ȼ�ѧ����ʽ����д���⣬�����Ѷ��еȣ�ע��ȷ�����к��Ⱥ�ȼ���ȵĸ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ���³�ѹ�£�11.2L�������е�ԭ����ĿΪNA | |
| B�� | ���³�ѹ�£�1.06g Na2CO3���е�Na+������Ϊ0.02 NA | |
| C�� | �ڱ�״���£�22.4 Lˮ������ԼΪ18g | |
| D�� | ���ʵ���Ũ��Ϊ0.5mol/L��MgCl2��Һ�У�����Cl-����Ϊ NA |
| A�� | 6.8 g���ڵ�KHSO4�к���0.05NA�������� | |
| B�� | 1.0 L 2 mol/L��NaOH��aq���к��е���ԭ����ĿΪ2NA | |
| C�� | ��24 g18O2�к���1.5NA����ԭ�� | |
| D�� | �ڷ�ӦKIO3+6HI=KI+3I2+3H2O�У�ÿ����3 mol I2ת�Ƶĵ�����Ϊ6NA |
| A�� | 1 mol H2O���е�ԭ����ΪNA | |
| B�� | 4g H2�����������4NA | |
| C�� | 1L 0.1 mol•L-1NaCl��Һ�к�Na+��0.1NA | |
| D�� | ���³�ѹ�£�11.2L O2�к��з�����Ϊ0.5NA |
| A�� | �������� | B�� | �⻯�� | C�� | ������ | D�� | �Ȼ��� |
| A�� | �ɵ������MgCl2����ȡ����þ��Ҳ�ܵ������HCl����ȡCl2 | |
| B�� | ±��Ԫ�صĵ��ʴ�F2��I2���۵������ߣ�������Ԫ�ص��ʴ�Li��Cs���۵�Ҳ������ | |
| C�� | ��2����Ԫ���⻯���ȶ���˳����HF��H2O��NH3�����������Ԫ���⻯���ȶ���˳����HCl��H2S��PH3 | |
| D�� | ������Cl2ͨ��FeBr2��Һ������FeCl3��FeBr3����������Cl2ͨ��FeI2 ��Һ��Ҳ����FeCl3��FeI3 |
| A�� | ��ȥKCl�л��е�KI������ˮͨ����������������ᾧ | |
| B�� | ��ȥBaSO4�����л��е�BaCO3���ӹ���������� | |
| C�� | ��ȥNa2CO3�����е�NaHCO3������������ | |
| D�� | ��ȥCuO�л��е�Al2O3���ӹ���������� |
 ��
��