��Ŀ����
����Ŀ����֪W��X��Y��ZΪ������Ԫ�أ�W��Zͬ���壬X��Y��Zͬ���ڣ�W����̬�⻯����ȶ��Դ���Z����̬�⻯����ȶ��ԣ�X��YΪ����Ԫ�أ�X�������ӵ�������С��Y�������ӵ������ԡ�����˵����ȷ���ǣ� ��
A.W��XԪ�صĵ����ڳ����²���Ӧ
B.X��Y��Z��W��ԭ�Ӱ뾶���μ�С
C.W����̬�⻯��ķе�һ������Z����̬�⻯��ķе�
D.��W��Y��ԭ���������5��������γɻ�����Ļ�ѧʽһ��ΪY2W3
���𰸡�B
��������
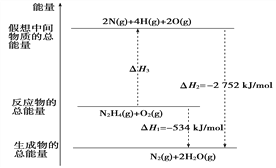
W��X��Y��ZΪ������Ԫ�أ�W��Zͬ���壬W����̬�⻯����ȶ��Ա�Z����̬�⻯����ȶ���ǿ����W��ZΪ�ǽ�����ԭ������Z��W��W���ڵڶ����ڣ�Z���ڵ������ڣ�X��Y��Zͬ���ڣ�X��YΪ����Ԫ�أ�X�������ӵ�������С��Y�������ӵ������ԣ���ԭ������Y>X���Ҷ��ߴ��ڵ������ڣ�X��Y��Z��ԭ������Z��Y��X���ݴ˷�����
W��X��Y��ZΪ������Ԫ�أ�W��Zͬ���壬W����̬�⻯����ȶ��Ա�Z����̬�⻯����ȶ���ǿ����W��ZΪ�ǽ�����ԭ������Z��W��W���ڵڶ����ڣ�Z���ڵ������ڣ�X��Y��Zͬ���ڣ�X��YΪ����Ԫ�أ�X�������ӵ�������С��Y�������ӵ������ԣ���ԭ������Y>X���Ҷ��ߴ��ڵ������ڣ�X��Y��Z��ԭ������Z��Y��X��
A����WΪ����XΪ�ƣ�����߳����¿ɷ�����Ӧ���������ƣ���A����
B��W��Zͬ������Wλ��Z�Ϸ���ԭ�Ӱ뾶Z��W��X��Y��Zͬ������ԭ�������������뾶���μ�С������ԭ�Ӱ뾶X��Y��Z��W����B��ȷ��
C��W����̬�⻯��Ϊ������ˮ��������ʱ�����Ӽ����������е����ͬ�������⻯��ķе㣬�������������ڷ��Ӿ��壬��Է�������Խ�е�Խ�ߣ�WΪC��ZΪSiʱ��W�⻯��е�ϵͣ���C����
D����WΪNԪ�أ�YΪMgԪ�أ����ߵ�ԭ���������5�������γɻ�����Ļ�ѧʽ����Ϊ![]() ��ΪY3W2����D����
��ΪY3W2����D����
�ʴ�ѡB��

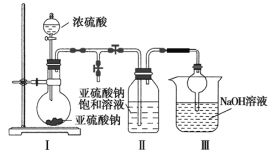
����Ŀ���ö��Ե缫��ⱥ��ʳ��ˮ��������Ca2+��Mg2+�����������ʵ�飬���һ��ʱ�������װ�ü���Ӧ���������£�
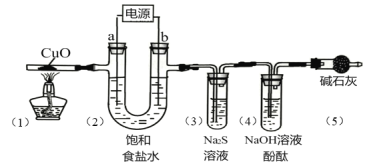
��Ӧ���� | ��1���к�ɫ������ | ��2���缫a������Һ���ֻ��� | ��3������Һ���ֻ��� | ��4������Һ��ɫ��ȥ |
���ж�ʵ�������������ȷ���ǣ� ��
A.��1����CuO��H2![]() Cu��H2O
Cu��H2O
B.��2����a�缫��2H2O��2e-=H2��+2OH-��Mg2+��2OH-=Mg(OH)2��
C.��3����Cl2��S2-=S��+2Cl-
D.��4����Cl2��H2O![]() HCl + HClO
HCl + HClO
����Ŀ������һ�������£������Ϊ5 L���ܱ������У�A��B��C������������ʵ���n(mol)��ʱ��t(min)�ı仯��ͼ��ʾ����֪��ƽ������¶ȣ�A�������������С��
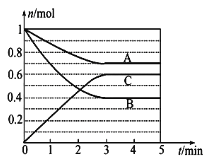
(1)�÷�Ӧ�Ļ�ѧ����ʽΪ__________��
(2)�÷�Ӧ�ķ�Ӧ����v��ʱ��t�Ĺ�ϵ��ͼ��ʾ��
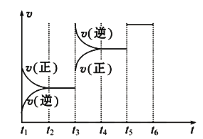
��������ͼ�жϣ���t3ʱ�̸ı�����������____________��
��A��ת��������һ��ʱ����________��
�����ε�ƽ�ⳣ�����±���ʾ��
t2��t3 | t4��t5 | t5��t6 |
K1 | K2 | K3 |
K1��K2��K3֮��Ĵ�С��ϵΪ________(����>������<��������������)��
�������ܱ������г���һ������H2S��������Ӧ2H2S(g) ![]() 2H2(g)��S2(g)����H����ͼ��ʾΪH2S����ֽ�����H2(g)��S2(g)��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��
2H2(g)��S2(g)����H����ͼ��ʾΪH2S����ֽ�����H2(g)��S2(g)��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��
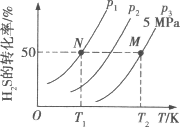
(1)��H___ (����>����<������ = ��)0��
(2)ͼ��ѹǿ(p1��p2��p3)�Ĵ�С˳��Ϊ____��
(3)ͼ��M���ƽ�ⳣ��Kp =____MPa(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ����ѹ�����ʵ�������)
(4)������һ�����H2S��ת����,���ı��¶ȡ�ѹǿ�⣬�����Բ�ȡ�Ĵ�ʩ��___��