��Ŀ����
����Ŀ��
���������������ճ�����������Ӧ�ù㷺�IJ��ϡ���ش��������⣺
��l����̬��ԭ�ӵļ۵��ӹ������ʽΪ__________��
��2����Ԫ�س�����������Fe2+��Fe3+���ȶ���Fe2+_______Fe2+����������������С����)��ԭ����________________��
��3�������������ܴ�����ƽ���NH4ClO4�ķֽ⣬NH4+�ĽṹʽΪ______�������λ�������ռ乹��Ϊ_________�����е�ԭ�ӵ��ӻ���ʽΪ_______����ClO4-��Ϊ�ȵ�����ķ��ӻ�������__________����д���֣���
��4������������ԭ�Ӳ���________�ѻ���������Ŀռ�������Ϊ______���ú�����ʽ�ӱ�ʾ����
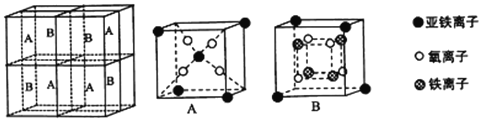
��5��ij�����������������ᄃ����ͼ��ʾ������A��B������ɡ����Ȩ������Fe2+��Fe3+��O2-�ĸ�����Ϊ_______������������ȣ�����֪�þ�����ܶ�Ϊdg/cm3�������ӵ�������ֵΪNA����Ʒ������a Ϊ_______nm���ú�d ��NA�Ĵ���ʽ��ʾ����
���𰸡� 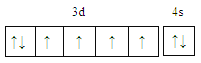 С�� Fe2+�ļ۵����Ų�ʽΪ3d6��Fe3+�ļ۵����Ų�ʽΪ3d5��Fe3+ ��3d�ܼ�Ϊ����״̬���ȶ���
С�� Fe2+�ļ۵����Ų�ʽΪ3d6��Fe3+�ļ۵����Ų�ʽΪ3d5��Fe3+ ��3d�ܼ�Ϊ����״̬���ȶ��� ![]() ���������� sp3�ӻ� CCl4��PO43- �����������𰸾��ɣ� ��������
���������� sp3�ӻ� CCl4��PO43- �����������𰸾��ɣ� �������� ![]() 1:2:4
1:2:4 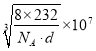
�����������������������Ҫ����ԭ�ӽṹ�뾧��ṹ��
��l����̬��ԭ�ӵļ۵��ӹ������ʽΪ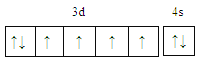 ��
��
��2����Ԫ�س�����������Fe2+��Fe3+���ȶ���Fe2+Fe2+����������������С����)��ԭ����Fe2+�ļ۵����Ų�ʽΪ3d6��Fe3+�ļ۵����Ų�ʽΪ3d5��Fe3+ ��3d�ܼ�Ϊ����״̬���ȶ�����
��3�������������ܴ�����ƽ���NH4ClO4�ķֽ⣬NH4+�ĽṹʽΪ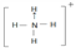 ���ռ乹��Ϊ���������Σ����е�ԭ�ӵļ۲���Ӷ�Ϊ4���ӻ���ʽΪsp3�ӻ���ClO4-��5ԭ�ӡ�32�۵��ӵ����ӣ���ClO4-��Ϊ�ȵ�����ķ��ӻ�������CCl4��PO43-��
���ռ乹��Ϊ���������Σ����е�ԭ�ӵļ۲���Ӷ�Ϊ4���ӻ���ʽΪsp3�ӻ���ClO4-��5ԭ�ӡ�32�۵��ӵ����ӣ���ClO4-��Ϊ�ȵ�����ķ��ӻ�������CCl4��PO43-��
��4������������ԭ�Ӳ������������ѻ���������ľ�������2����ԭ�ӣ��辧���߳�Ϊa������ԭ�Ӱ뾶Ϊr��
����Խ��߳�![]() a����Խ��߳�
a����Խ��߳�![]() a=4r����r=
a=4r����r=![]() ���ռ�������=
���ռ�������=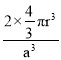 =
= ![]() ��
��
��5��A����1.5���������ӡ�4�������ӣ�B����0.5���������ӡ�4�������ӡ�4�������ӣ������������Fe2+��Fe3+��O2-�ĸ�����Ϊ1:2:4��
��������Fe2+��Fe3+��O2-�ĸ����ֱ���Ϊ4��8��16�����ǵ��������֮����8��232������m=��V�ɵ�8��232 g=d��g/cm3a3��NA��a =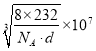 nm��
nm��