��Ŀ����
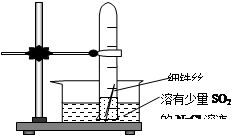
����Fe��OH��2���ױ�����������ʵ���Һ�������������Һ���ռӦ�Ƶð�ɫ������Fe��OH��2������Ӧ����ͼ��ʾװ�ÿ����Ƶð�ɫ������Fe��OH��2�������������Ϸֱ�Ϊ����ʯī��
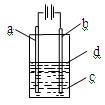
��1��a�缫����ӦΪ ���缫��ӦʽΪ ��
��2�����Һc����� �����ţ���
��3��dΪ������������ ���ڼ��뱽֮ǰ��
��cӦ���μ����� ��

��1��a�缫����ӦΪ ���缫��ӦʽΪ ��
��2�����Һc����� �����ţ���
| A����ˮ | B��AgNO3��Һ | C��NaOH��Һ | D��CuCl2��Һ |
��cӦ���μ����� ��
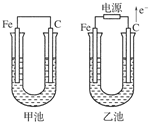
��1��Fe Fe-2e-= Fe2+
��2��C
��3���������� �������
��2��C
��3���������� �������
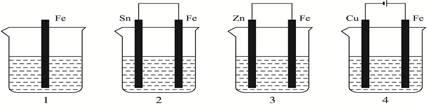
��ȡFe��OH��2�ܹؼ����ڷ�ֹ��������������
��1����ȡFe��OH��2�������ṩFe2+����a��Ӧ��������������ʧ���ӣ�Fe-2e-= Fe2+
��2������ˮ�ĵ����Խϲ�ų�A��ͬʱΪ�˵õ������ij������ų�BD��C��������
��3��ˮ�ϲ�ı����ɽϺõĸ�������������Ա���c��Һ���ܽ����������ɽ���������
��1����ȡFe��OH��2�������ṩFe2+����a��Ӧ��������������ʧ���ӣ�Fe-2e-= Fe2+
��2������ˮ�ĵ����Խϲ�ų�A��ͬʱΪ�˵õ������ij������ų�BD��C��������
��3��ˮ�ϲ�ı����ɽϺõĸ�������������Ա���c��Һ���ܽ����������ɽ���������

��ϰ��ϵ�д�
�����Ŀ
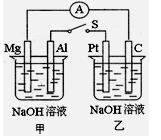
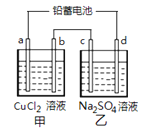
 2 PbSO4 (s) ��2 H2O (l) ���һ��ʱ�����c����d�������ֱ�μӷ�̪�Լ���c��������Һ��죬����˵����ȷ���ǣ� ��
2 PbSO4 (s) ��2 H2O (l) ���һ��ʱ�����c����d�������ֱ�μӷ�̪�Լ���c��������Һ��죬����˵����ȷ���ǣ� ��