��Ŀ����
����Ŀ����ͼ�ǽ�����������ʵ��ij���װ�ã����ж��й�ʵ�������������������ȷ����

A. ��ˮ����ʢ��ˮ���Թ���ʢ��SO2���ɿ����Թ���Һ������
B. ��ˮ����ʢ��ˮ���Թ���ʢ��NO2���ɿ����Թ���Һ�������������Թ�
C. ��ˮ����ʢ��ˮ�����з�̪�����Թ�����NH3���ɿ����Թ���Һ���������ʺ�ɫ
D. ��ˮ����ʢ��NaOH��Һ���Թ�����Cl2���ɿ����Թ���Һ������������ɫ��ȥ
���𰸡�B
��������
A����������������ˮ������ˮ�Ķ���������ˮ��Ӧ���������
B������������ˮ��Ӧ���������NO��NO������ˮ��
C��������������ˮ������ˮ�İ���������ˮ��Ӧ����һˮ�ϰ���
D��������NaOH��Һ��Ӧ����NaCl��NaClO��ˮ��
A���������������ˮ������ˮ�Ķ���������ˮ��Ӧ���������ᣬ����Կ���Һ����������A��ȷ��
B�����������ˮ��Ӧ���������NO��NO������ˮ����Һ����������ˮ������������Թܣ���B����
C�������������ˮ������ˮ�İ���������ˮ��Ӧ����һˮ�ϰ���һˮ�ϰ�����ʹ��Һ�Լ��ԣ�����̪��죬��Һ���������ʺ�ɫ����C��ȷ��
D�������NaOH��Һ��Ӧ����NaCl��NaClO��ˮ����Һ���������Թ��л���ɫ��ȥ����D��ȷ��
��ѡB��

 ��ǰ����ϵ�д�
��ǰ����ϵ�д�����Ŀ��ijС��ͬѧΪ̽�� H2O2�� H2SO3�� Br2 ������ǿ�����������ʵ�飨�г���������ȥ��װ�õ��������Ѽ��飩��
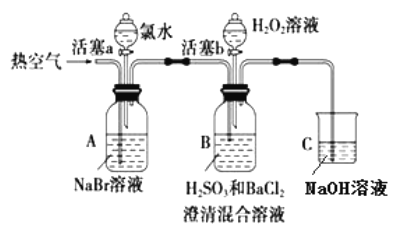
ʵ���¼���£�
ʵ����� | ʵ������ | |
�� | ���� a���μ���ˮ���رջ��� a | _____________________________________________ |
�� | �����ȿ���һ��ʱ���ֹͣ | A����Һ��ɫ���Ա�dz��B�������ݣ�����������ɫ�������������ϲ���ҺΪ��ɫ |
�� | ����b����μ���H2O2��Һ | ��ʼʱ��Һ��ɫ�����Ա仯�������μ�H2O2��Һ��һ��ʱ����Һ��ɳȺ�ɫ�� |
���������գ�
��1���ڽ��в�����ʱ��A�е�ʵ��������___________���йط�Ӧ�����ӷ���ʽ��___________��
��2�������ڴ����ȿ�����Ŀ����____________��B �в�����ɫ�����Ļ�ѧʽ��___________��
��3��װ��C��������____________________��
��4��������ʵ���֪���ڴ�ʵ�������£�H2O2��H2SO3��Br2������ǿ��˳��Ϊ________________��
��5�������ۿ�ʼʱ��ɫ�����Ա仯����ԭ���ǣ�д��һ�����ɣ���___________________��
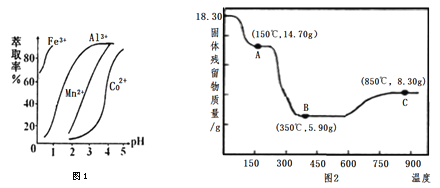
����Ŀ�������ܿ�����ָʾ���ʹ������Ʊ�����ˮ�ܿ���Ҫ�ɷ�ΪCo2O3��������Fe2O3��A12O3��MnO��MgO��CaO��SiO2�ȣ���ȡCoC2O4��2H2O�����������£�

��֪���ٽ���Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Ca2+��Mg2+��Al3+�ȣ�
�����������£�ClO3-��������Co2+��ClO3-ת��ΪCl-��
�۲���������������������ʽ����ʱ��Һ��pH������
������ | Fe(OH)3 | Al(OH)3 | Co(OH)2 | Fe(OH)2 | Mn(OH)2 |
��ȫ������pH | 3.7 | 5.2 | 9.2 | 9.6 | 9.8 |
��1�����������м���Na2SO3����ҪĿ����________��
��2�������Һ�м���NaClO3�����ӷ�Ӧ����ʽ��_________��
��3����֪��������NH3��H2O![]() NH4+��OH- Kb��1.8��10-5
NH4+��OH- Kb��1.8��10-5
H2C2O4![]() H+��HC2O4- Ka1��5.4��10-2
H+��HC2O4- Ka1��5.4��10-2
HC2O4-![]() H��C2O42- Ka2��5.4��10-5
H��C2O42- Ka2��5.4��10-5
�������������(NH4)2C2O4��Һ��pH______7�������������=������
��4������(NH4)2C2O4 ��Һ���������壬�ٹ��ˡ�ϴ�ӣ�ϴ��ʱ��ѡ�õ��Լ��У�________��
A������ˮ B������ˮ C�����͵�(NH4)2C2O4��Һ D��ϡ����
��5����ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ����ͼ1����ȡ����������________����ʹ�õ�����pH��Χ��________��
A��2.0��2.5 B��3.0��3.5 C��4.0��4.5
��6��CoC2O4��2H2O�ȷֽ������仯������ͼ2��ʾ������600����ǰ�Ǹ����������ȣ�600 ���Ժ����ڿ����м��ȡ�A��B��C��Ϊ�����C����ʾ����Ļ�ѧʽ��________��