��Ŀ����
����ѧ�D�Dѡ��ѧ�뼼����(15�֣�
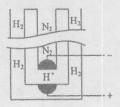
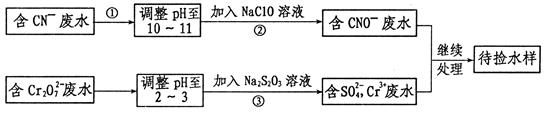
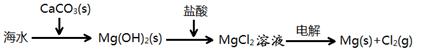
���ڹ�����ռ����Ҫ��λ����ͼ�Ǻϳɰ��ļ�Ҫ���̣�
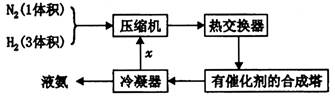
I.ԭ�������Ʊ���
(1) �ϳɰ����赪�����Կ���������֮һ�ǽ�����Һ�����ټ��ȷֹݣ��������һ�ִӿ����з���������ķ�����_________________________________________________________��
(2) ��д����ҵ�ϻ��������һ�ַ������û�ѧ����ʽ��ʾ��____________
II.ԭ�����ľ�������
Ϊ��ֹ�������ж�����ԭ�����ڽ���ѹ����֮ǰ���뾭�����������ƴ����������ơ�����ͨ���ǽ���������CO��CO2��O2��H2S�����ʵ�ԭ������ͨ�뺬�а�ˮ�Ĵ�����ͭ������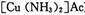 )��Һ���Ի�ô���ԭ���������У�����CO�ķ�ӦΪ��
)��Һ���Ի�ô���ԭ���������У�����CO�ķ�ӦΪ��
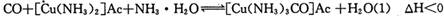
(3) Ϊ���{CO�����ʣ��ɲ�ȡ����Ч��ʩ��__________________
(4) ��ȥ����ʱ��������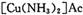 ����Ϊ
����Ϊ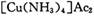 ����Ӧ�л�ԭ���������������ʵ���֮����____________��
����Ӧ�л�ԭ���������������ʵ���֮����____________��

III.���ĺϳɣ�
(5)�ݱ�������ѧ�Ҳ��ø����ӵ����Ե�SCY�մ�(�ܴ���H+)Ϊ���ʣ�������������������ϵĽ����ٶྦྷ��Ĥ���缫��ʵ�����{�³�ѹ�¸�ת���ʵĵ绯ѧ�ϳɰ�����ʵ��װ����ͼ����д���ٵ缫A�ϵĵ缫��Ӧʽ________________________
���ڹ�����ռ����Ҫ��λ����ͼ�Ǻϳɰ��ļ�Ҫ���̣�

I.ԭ�������Ʊ���
(1) �ϳɰ����赪�����Կ���������֮һ�ǽ�����Һ�����ټ��ȷֹݣ��������һ�ִӿ����з���������ķ�����_________________________________________________________��
(2) ��д����ҵ�ϻ��������һ�ַ������û�ѧ����ʽ��ʾ��____________
II.ԭ�����ľ�������
Ϊ��ֹ�������ж�����ԭ�����ڽ���ѹ����֮ǰ���뾭�����������ƴ����������ơ�����ͨ���ǽ���������CO��CO2��O2��H2S�����ʵ�ԭ������ͨ�뺬�а�ˮ�Ĵ�����ͭ������
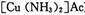 )��Һ���Ի�ô���ԭ���������У�����CO�ķ�ӦΪ��
)��Һ���Ի�ô���ԭ���������У�����CO�ķ�ӦΪ��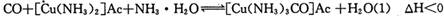
(3) Ϊ���{CO�����ʣ��ɲ�ȡ����Ч��ʩ��__________________
(4) ��ȥ����ʱ��������
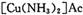 ����Ϊ
����Ϊ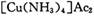 ����Ӧ�л�ԭ���������������ʵ���֮����____________��
����Ӧ�л�ԭ���������������ʵ���֮����____________��
III.���ĺϳɣ�
(5)�ݱ�������ѧ�Ҳ��ø����ӵ����Ե�SCY�մ�(�ܴ���H+)Ϊ���ʣ�������������������ϵĽ����ٶྦྷ��Ĥ���缫��ʵ�����{�³�ѹ�¸�ת���ʵĵ绯ѧ�ϳɰ�����ʵ��װ����ͼ����д���ٵ缫A�ϵĵ缫��Ӧʽ________________________
(1)��̼�ڿ�����ȼ�գ�ʹ�����ľ�����ͨ����Һ��ȥCO2�ɵõ�������
(2) C+H2O CO+H2����CO+H2O
CO+H2����CO+H2O CO2+H2����CH4
CO2+H2����CH4 C+2H2����CH4+H2O
C+2H2����CH4+H2O  CO +3H2
CO +3H2
(3)���¡���ѹ(4) 4��1��(5) N2+6e-+6H+=2NH3��
(2) C+H2O
 CO+H2����CO+H2O
CO+H2����CO+H2O CO2+H2����CH4
CO2+H2����CH4 C+2H2����CH4+H2O
C+2H2����CH4+H2O  CO +3H2
CO +3H2(3)���¡���ѹ(4) 4��1��(5) N2+6e-+6H+=2NH3��
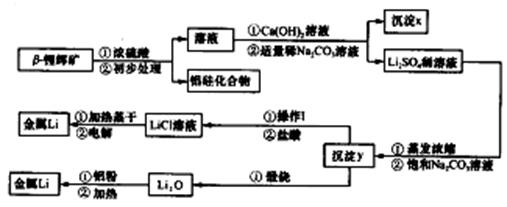
���⿼��ϳɰ����й�֪ʶ��(1) ������Ҫ�������͵����Ļ������Բ��û�ѧ������ȥ�������õ���������������̼�ڿ�����ȼ�գ�ʹ�����ľ�����ͨ����Һ��ȥCO2�ɵõ�������
(2) ������ͨ����ˮ����ͨ�����ȵ�̼��õ�����������ʽΪC+H2O CO+H2����CO+H2O
CO+H2����CO+H2O CO2+H2������ͨ������ĸ��·ֽ�õ�CH4
CO2+H2������ͨ������ĸ��·ֽ�õ�CH4 C+2H2���������ˮ������Ӧ�õ���CH4+H2O
C+2H2���������ˮ������Ӧ�õ���CH4+H2O  CO +3H2��
CO +3H2��
(3) ����CO�Ŀ��淴Ӧ����Ӧ�������С�����ȷ�Ӧ����˿��Բ��ý��ºͼ�ѹ�ķ������������CO��Ч�ʡ�
(4) �е�Cu��+1�ۣ�
�е�Cu��+1�ۣ�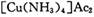 �е�Cu��+2�ۣ���1omlO2Ҫ�õ�4oml���ӣ����ݵ�ʧ�������غ㣬��Ӧ�л�ԭ���������������ʵ���֮��4��1��
�е�Cu��+2�ۣ���1omlO2Ҫ�õ�4oml���ӣ����ݵ�ʧ�������غ㣬��Ӧ�л�ԭ���������������ʵ���֮��4��1��
(5) ��ʵ��װ����ͼ���Կ������绯ѧ�ϳɰ���ͨ��ϳɣ�A�缫��N2�õ����ӣ�������ԭ��Ӧ���缫��ӦʽΪN2+6e-+6H+=2NH3��
(2) ������ͨ����ˮ����ͨ�����ȵ�̼��õ�����������ʽΪC+H2O
 CO+H2����CO+H2O
CO+H2����CO+H2O CO2+H2������ͨ������ĸ��·ֽ�õ�CH4
CO2+H2������ͨ������ĸ��·ֽ�õ�CH4 C+2H2���������ˮ������Ӧ�õ���CH4+H2O
C+2H2���������ˮ������Ӧ�õ���CH4+H2O  CO +3H2��
CO +3H2��(3) ����CO�Ŀ��淴Ӧ����Ӧ�������С�����ȷ�Ӧ����˿��Բ��ý��ºͼ�ѹ�ķ������������CO��Ч�ʡ�
(4)
 �е�Cu��+1�ۣ�
�е�Cu��+1�ۣ�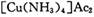 �е�Cu��+2�ۣ���1omlO2Ҫ�õ�4oml���ӣ����ݵ�ʧ�������غ㣬��Ӧ�л�ԭ���������������ʵ���֮��4��1��
�е�Cu��+2�ۣ���1omlO2Ҫ�õ�4oml���ӣ����ݵ�ʧ�������غ㣬��Ӧ�л�ԭ���������������ʵ���֮��4��1��(5) ��ʵ��װ����ͼ���Կ������绯ѧ�ϳɰ���ͨ��ϳɣ�A�缫��N2�õ����ӣ�������ԭ��Ӧ���缫��ӦʽΪN2+6e-+6H+=2NH3��

��ϰ��ϵ�д�
�����Ŀ
 �� 400�桢30MPa��n(NH3)��n(H2)
�� 400�桢30MPa��n(NH3)��n(H2)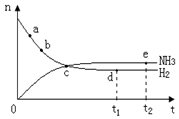
 Si + CO2��
Si + CO2��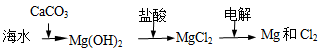
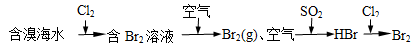
 �еĻ�ԭ����Ҫ��CO
�еĻ�ԭ����Ҫ��CO 2NH3��g�� ��H=-92��44 kJ��mol���䲿�ֹ�����������ͼ��ʾ��
2NH3��g�� ��H=-92��44 kJ��mol���䲿�ֹ�����������ͼ��ʾ��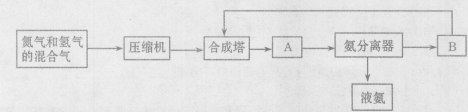
 ��������ӦΪ ��
��������ӦΪ ��