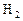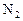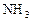��Ŀ����
��ҵ�Ϻϳɰ���һ�������½������·�Ӧ��N2��g��+3H2��g��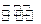 2NH3��g�� ��H=-92��44 kJ��mol���䲿�ֹ�����������ͼ��ʾ��
2NH3��g�� ��H=-92��44 kJ��mol���䲿�ֹ�����������ͼ��ʾ��
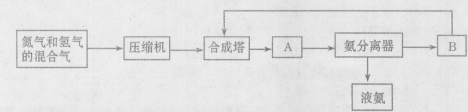
��1���ϳɰ�����Ҫ��ԭ�����У�����ȡ�� ��������Դ�� ��
��2����ԭ�������о���������Ŀ���� ��
��3���豸A�������� ���豸B�������� ��
��4����20��50 Mpaʱ����ҵ�ϳɰ�ѡ����400��500����¶Ƚ��з�Ӧ��
��Ҫԭ���� ��
��5���ݡ���ѧ����־������ϣ����ѧ���ڳ�ѹ�½�������������ϡ�͵ĵ����ֱ�ͨ��һ�����ȵ�570��ĵ��أ���ͼ���У���͵��ڵ缫�Ϻϳ��˰�����ת���ʴﵽ��78������������ӦΪ ��������ӦΪ ��
��������ӦΪ ��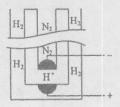
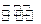 2NH3��g�� ��H=-92��44 kJ��mol���䲿�ֹ�����������ͼ��ʾ��
2NH3��g�� ��H=-92��44 kJ��mol���䲿�ֹ�����������ͼ��ʾ��
��1���ϳɰ�����Ҫ��ԭ�����У�����ȡ�� ��������Դ�� ��
��2����ԭ�������о���������Ŀ���� ��
��3���豸A�������� ���豸B�������� ��
��4����20��50 Mpaʱ����ҵ�ϳɰ�ѡ����400��500����¶Ƚ��з�Ӧ��
��Ҫԭ���� ��
��5���ݡ���ѧ����־������ϣ����ѧ���ڳ�ѹ�½�������������ϡ�͵ĵ����ֱ�ͨ��һ�����ȵ�570��ĵ��أ���ͼ���У���͵��ڵ缫�Ϻϳ��˰�����ת���ʴﵽ��78������������ӦΪ
 ��������ӦΪ ��
��������ӦΪ ��

��

��ϰ��ϵ�д�
�����Ŀ

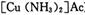 )��Һ���Ի�ô���ԭ���������У�����CO�ķ�ӦΪ��
)��Һ���Ի�ô���ԭ���������У�����CO�ķ�ӦΪ��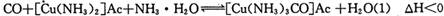
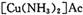 ����Ϊ
����Ϊ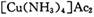 ����Ӧ�л�ԭ���������������ʵ���֮����____________��
����Ӧ�л�ԭ���������������ʵ���֮����____________��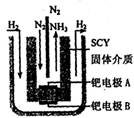
 ���Ʊ�H2
���Ʊ�H2
 ������ͼ��д����ͼ�Т٢ڵĻ�ѧʽ���� ���� ���������з����Ļ�ѧ��Ӧ����ʽΪ ��
������ͼ��д����ͼ�Т٢ڵĻ�ѧʽ���� ���� ���������з����Ļ�ѧ��Ӧ����ʽΪ ��
 4 SiHCl3��g������ƽ���H2��SiHCl3���ʵ���Ũ�ȷֱ�Ϊ0.140mol/L��0.020mol/L����H2ȫ����Դ���ȼҵ�������������Ĵ�NaCl��
4 SiHCl3��g������ƽ���H2��SiHCl3���ʵ���Ũ�ȷֱ�Ϊ0.140mol/L��0.020mol/L����H2ȫ����Դ���ȼҵ�������������Ĵ�NaCl�� ����Ϊ kg��
����Ϊ kg��
 �ɡ���֪�����£�Al(OH)3��Һ��pH=3����Ksp=1��10-36��������c(Al3+)�� �� ��
�ɡ���֪�����£�Al(OH)3��Һ��pH=3����Ksp=1��10-36��������c(Al3+)�� �� ��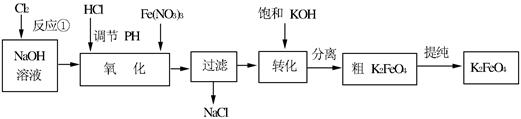
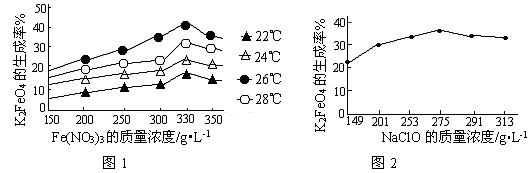
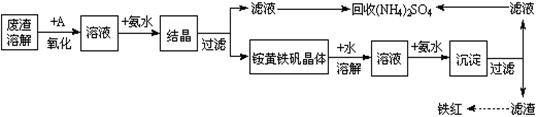
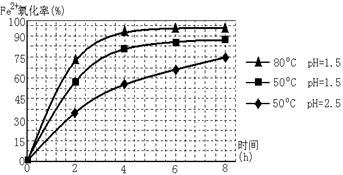
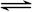 4Fe(OH)3+8OH��+3O2�����ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ��_______��Һ������ţ���
4Fe(OH)3+8OH��+3O2�����ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ��_______��Һ������ţ���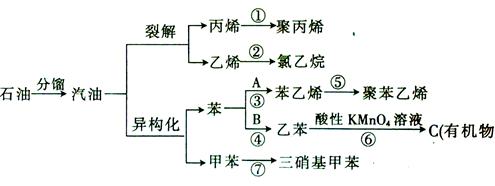
 ____________________________________________��
____________________________________________��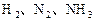 ��Ӧ��ƽ��ʱ������Ũ������һ���ǣ� ��
��Ӧ��ƽ��ʱ������Ũ������һ���ǣ� ��