��Ŀ����
����Ŀ������˵����ȷ����![]()
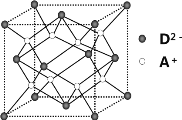
A.��ѿ�������ǵ�ˮ�������������ǣ��ʶ��߾�Ϊ��ԭ�Ͷ���
B.�����£���![]() HA��Һ��
HA��Һ��![]() ��Һ��������
��Һ��������![]() ��������ı仯
��������ı仯![]() ��û����Һ��
��û����Һ��![]() ��������Һ����ˮ�������
��������Һ����ˮ�������![]()
C.��̼�²�����̼������ĭ����ÿ����ĭ����Լ4000��̼ԭ�ӣ�ֱ��Լ6��9nm���ڵ���![]() ʱ����ĭ�������ô��ԣ���̼������ĭ����ʯī��Ϊͬ��������
ʱ����ĭ�������ô��ԣ���̼������ĭ����ʯī��Ϊͬ��������
D.��֪![]() ��
��![]() Ϊ
Ϊ![]() ���������
���������![]()
![]() ��
��![]() ��Һ��
��Һ��![]()
![]()
![]() ��Һ��Ϻ����
��Һ��Ϻ����![]() ��������
��������
���𰸡�C
��������
A. ����ˮ���IJ����������л�ԭ�ԣ�����û�л�ԭ�ԣ����Բ��ǻ�ԭ�Ͷ��ǣ���A����
B. ��û����Һ��pH=5,HA����,��Һ��ʾ����,��Ӧ��Ļ��Һ��������������ˮ�����,����ˮ�����������Ũ��Ϊ��![]() ����B����
����B����
C. ��̼������ĭ������̼������ʯī��Ϊͬ�������壬��C��ȷ��
D. �������Ϻ�,![]() ,
,![]() ,
,![]() ��
��
��û�г�����������D����
��ѡC.

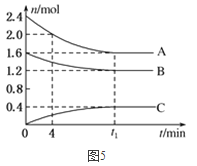
����Ŀ������Dz�ͬ�¶���ˮ�����ӻ����ݣ�
�¶� | 25 |
|
|
ˮ�����ӻ����� |
|
|
|
�Իش��������⣺
![]() ��
��![]() ����
����![]() __________
__________![]() ����
����![]() ������
������![]() ������
������![]() ��
��![]() ���������жϵ�������__________��
���������жϵ�������__________��
![]() �£�ij
�£�ij![]() ��Һ��
��Һ��![]() ��ȡ����Һ
��ȡ����Һ![]() ����ˮϡ����
����ˮϡ����![]() ����ϡ�ͺ���Һ��
����ϡ�ͺ���Һ��![]() span>__________��
span>__________��
![]() �£���
�£���![]() �Ŀ�������Һ
�Ŀ�������Һ![]() ��
��![]() ��ϡ����
��ϡ����![]() ���
���![]() ���Ϻ���Һ�����Ϊԭ����Һ���֮��
���Ϻ���Һ�����Ϊԭ����Һ���֮��![]() �����û����Һ��
�����û����Һ��![]() ����
����![]() __________������Һ�и������ӵ�Ũ���ɴ�С������˳����__________________��
__________������Һ�и������ӵ�Ũ���ɴ�С������˳����__________________��
![]() �ֱ�����������ͬpH��HCl��Һ��
�ֱ�����������ͬpH��HCl��Һ��![]() ��Һ�м���������Zn�ۣ���Ӧ�տ�ʼʱ����
��Һ�м���������Zn�ۣ���Ӧ�տ�ʼʱ����![]() �����ʣ�
�����ʣ�![]() ______
______![]() ����
����![]() ������
������![]() ������
������![]() ����ͬ
����ͬ![]() ����Ӧ��ȫ������������������
����Ӧ��ȫ������������������![]() ����_______
����_______![]() ���ᡣ
���ᡣ