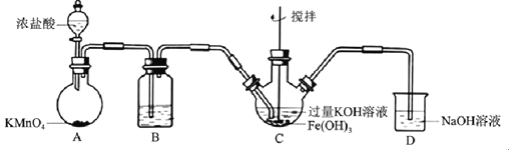
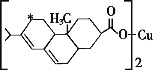
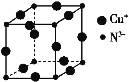
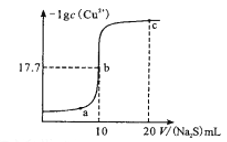
ĢāÄæÄŚČŻ
”¾ĢāÄæ”æČē±ķŹĒ²»Ķ¬ĪĀ¶ČĻĀĖ®µÄĄė×Ó»żŹż¾Ż£ŗ
ĪĀ¶Č | 25 |
|
|
Ė®µÄĄė×Ó»ż³£Źż |
|
|
|
ŹŌ»Ų“šŅŌĻĀĪŹĢā£ŗ
![]() Čō
Čō![]() £¬Ōņ
£¬Ōņ![]() __________
__________![]() Ģī”°
Ģī”°![]() ”±”¢”°
”±”¢”°![]() ”±»ņ”°
”±»ņ”°![]() ”±
”±![]() £¬×÷³ö“ĖÅŠ¶ĻµÄĄķÓÉŹĒ__________”£
£¬×÷³ö“ĖÅŠ¶ĻµÄĄķÓÉŹĒ__________”£
![]() ĻĀ£¬Ä³
ĻĀ£¬Ä³![]() ČÜŅŗÖŠ
ČÜŅŗÖŠ![]() £¬Č”øĆČÜŅŗ
£¬Č”øĆČÜŅŗ![]() £¬¼ÓĖ®Ļ”ŹĶÖĮ
£¬¼ÓĖ®Ļ”ŹĶÖĮ![]() £¬ŌņĻ”ŹĶŗóČÜŅŗÖŠ
£¬ŌņĻ”ŹĶŗóČÜŅŗÖŠ![]() span>__________”£
span>__________ӣ
![]() ĻĀ£¬½«
ĻĀ£¬½«![]() µÄæĮŠŌÄĘČÜŅŗ
µÄæĮŠŌÄĘČÜŅŗ![]() Óė
Óė![]() µÄĻ”ĮņĖį
µÄĻ”ĮņĖį![]() »ģŗĻ
»ģŗĻ![]() Éč»ģŗĻŗóČÜŅŗµÄĢå»żĪŖŌĮ½ČÜŅŗĢå»żÖ®ŗĶ
Éč»ģŗĻŗóČÜŅŗµÄĢå»żĪŖŌĮ½ČÜŅŗĢå»żÖ®ŗĶ![]() £¬ĖłµĆ»ģŗĻČÜŅŗµÄ
£¬ĖłµĆ»ģŗĻČÜŅŗµÄ![]() £¬Ōņ
£¬Ōņ![]() __________”£“ĖČÜŅŗÖŠø÷ÖÖĄė×ÓµÄÅضČÓɓ󵽊”µÄÅÅĮŠĖ³ŠņŹĒ__________________”£
__________”£“ĖČÜŅŗÖŠø÷ÖÖĄė×ÓµÄÅضČÓɓ󵽊”µÄÅÅĮŠĖ³ŠņŹĒ__________________”£
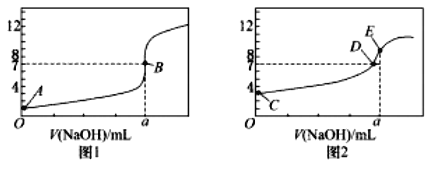
![]() ·Ö±šĻņµČĢå»ż”¢ĻąĶ¬pHµÄHClČÜŅŗŗĶ
·Ö±šĻņµČĢå»ż”¢ĻąĶ¬pHµÄHClČÜŅŗŗĶ![]() ČÜŅŗÖŠ¼ÓČė×ćĮæµÄZn·Ū£¬·“Ó¦øÕæŖŹ¼Ź±²śÉś
ČÜŅŗÖŠ¼ÓČė×ćĮæµÄZn·Ū£¬·“Ó¦øÕæŖŹ¼Ź±²śÉś![]() µÄĖŁĀŹ£ŗ
µÄĖŁĀŹ£ŗ![]() ______
______![]() Ģī”°
Ģī”°![]() ”±”¢”°
”±”¢”°![]() ”±»ņ”°
”±»ņ”°![]() ”±ĻĀĶ¬
”±ĻĀĶ¬![]() £¬·“Ó¦ĶźČ«ŗó£¬ĖłµĆĒāĘųµÄÖŹĮæ£ŗ
£¬·“Ó¦ĶźČ«ŗó£¬ĖłµĆĒāĘųµÄÖŹĮæ£ŗ![]() ŃĪĖį_______
ŃĪĖį_______![]() “×Ėį”£
“×Ėį”£
”¾“š°ø”æ![]() Ė®µÄµēĄėĪüČČ£¬ĪĀ¶ČÉżøߣ¬Ę½ŗāÕżĻņŅĘ¶Æ£¬KwŌö“ó£»
Ė®µÄµēĄėĪüČČ£¬ĪĀ¶ČÉżøߣ¬Ę½ŗāÕżĻņŅĘ¶Æ£¬KwŌö“ó£» ![]()
![]()
![]() £»
£» ![]()
![]()
”¾½āĪö”æ
![]() Ė®ŹĒČõµē½āÖŹ£¬“ęŌŚµēĄėĘ½ŗā£¬µēĄė¹ż³ĢĪŖĪüČČ·“Ó¦£¬ĖłŅŌĪĀ¶ČÉżøߣ¬Ė®µÄµēĄė³Ģ¶ČŌö“ó£¬Ąė×Ó»żŌö“ó£¬ĖłŅŌKw>1”Į10-14£¬
Ė®ŹĒČõµē½āÖŹ£¬“ęŌŚµēĄėĘ½ŗā£¬µēĄė¹ż³ĢĪŖĪüČČ·“Ó¦£¬ĖłŅŌĪĀ¶ČÉżøߣ¬Ė®µÄµēĄė³Ģ¶ČŌö“ó£¬Ąė×Ó»żŌö“ó£¬ĖłŅŌKw>1”Į10-14£¬
¹Ź“š°øĪŖ£ŗ![]() £»Ė®µÄµēĄėĪüČČ£¬ĪĀ¶ČÉżøߣ¬Ę½ŗāÕżĻņŅĘ¶Æ£¬KwŌö“ó£»
£»Ė®µÄµēĄėĪüČČ£¬ĪĀ¶ČÉżøߣ¬Ę½ŗāÕżĻņŅĘ¶Æ£¬KwŌö“ó£»
![]() Ź±£¬Ä³
Ź±£¬Ä³![]() ČÜŅŗÖŠc
ČÜŅŗÖŠc![]()
![]() £¬ŌņČÜŅŗÖŠÄĘĄė×ÓÅØ¶ČŹĒ
£¬ŌņČÜŅŗÖŠÄĘĄė×ÓÅØ¶ČŹĒ![]()
![]() Čē¹ūĻ”ŹĶ10±¶£¬ŌņÄĘĄė×ÓÅØ¶ČŹĒ
Čē¹ūĻ”ŹĶ10±¶£¬ŌņÄĘĄė×ÓÅØ¶ČŹĒ![]()
![]() µ«ĮņĖįÄĘČÜŅŗŹĒĻŌÖŠŠŌµÄ£¬c(OH-)=1”Į10-7mol/L£¬ĖłŅŌ
µ«ĮņĖįÄĘČÜŅŗŹĒĻŌÖŠŠŌµÄ£¬c(OH-)=1”Į10-7mol/L£¬ĖłŅŌ![]() £ŗ
£ŗ![]()
![]() £ŗ
£ŗ![]() £¬
£¬
¹Ź“š°øĪŖ£ŗ![]() £»
£»
![]() ČōĖłµĆ»ģŗĻŅŗµÄ
ČōĖłµĆ»ģŗĻŅŗµÄ![]() £¬Ėį¹żĮ棬
£¬Ėį¹żĮ棬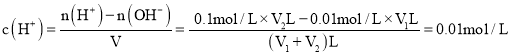 £¬½āÖ®µĆ£ŗ
£¬½āÖ®µĆ£ŗ![]() £ŗ
£ŗ![]() ”£¼ŁÉčNaOHČÜŅŗĪŖ9L£¬ŌņĮņĖįČÜŅŗĪŖ11L£¬NaOHµÄÅضČĪŖ0.1mol/L£¬ĮņĖįµÄÅضČĪŖ0.05mol/L£¬n(NaOH)=0.9mol£¬n(H2SO4)=0.55mol£¬ĖłŅŌn(Na+)>n(SO42-)£¬Į½ČÜŅŗ»ģŗĻŗó£¬Na+ŗĶSO42-µÄĪļÖŹµÄĮæ²»±ä£¬ĖłŅŌc(Na+)>c(SO42-)£¬ÓÖÓÉÓŚČÜŅŗĻŌĖįŠŌ£¬ĖłŅŌc(H+)>c(OH-)£¬ĖłŅŌČÜŅŗÖŠĄė×ÓÅضČÓɓ󵽊”µÄÅÅĮŠĖ³ŠņĪŖ
”£¼ŁÉčNaOHČÜŅŗĪŖ9L£¬ŌņĮņĖįČÜŅŗĪŖ11L£¬NaOHµÄÅضČĪŖ0.1mol/L£¬ĮņĖįµÄÅضČĪŖ0.05mol/L£¬n(NaOH)=0.9mol£¬n(H2SO4)=0.55mol£¬ĖłŅŌn(Na+)>n(SO42-)£¬Į½ČÜŅŗ»ģŗĻŗó£¬Na+ŗĶSO42-µÄĪļÖŹµÄĮæ²»±ä£¬ĖłŅŌc(Na+)>c(SO42-)£¬ÓÖÓÉÓŚČÜŅŗĻŌĖįŠŌ£¬ĖłŅŌc(H+)>c(OH-)£¬ĖłŅŌČÜŅŗÖŠĄė×ÓÅضČÓɓ󵽊”µÄÅÅĮŠĖ³ŠņĪŖ![]() £¬
£¬
¹Ź“š°øĪŖ£ŗ![]() £¬
£¬![]() £»
£»
![]() ĻąĶ¬µÄ²»Ķ¬ĖįÖŠ£¬ĒāĄė×ÓÅضČĻąĶ¬£¬ĖłŅŌ·“Ó¦øÕæŖŹ¼Ź±²śÉś
ĻąĶ¬µÄ²»Ķ¬ĖįÖŠ£¬ĒāĄė×ÓÅضČĻąĶ¬£¬ĖłŅŌ·“Ó¦øÕæŖŹ¼Ź±²śÉś![]() µÄĖŁĀŹĻąµČ£»“×ĖįÖŠĖįµÄÅØ¶Č“óÓŚĒāĄė×ÓÅØ¶Č£¬ŃĪĖįÖŠĖįµÄÅØ¶ČµČÓŚĒāĄė×ÓÅØ¶Č£¬ĖłŅŌ“×ĖįµÄÅØ¶Č“óÓŚHClµÄÅØ¶Č£¬ĖłŅŌĖłµĆĒāĘųµÄÖŹĮæ£ŗ
µÄĖŁĀŹĻąµČ£»“×ĖįÖŠĖįµÄÅØ¶Č“óÓŚĒāĄė×ÓÅØ¶Č£¬ŃĪĖįÖŠĖįµÄÅØ¶ČµČÓŚĒāĄė×ÓÅØ¶Č£¬ĖłŅŌ“×ĖįµÄÅØ¶Č“óÓŚHClµÄÅØ¶Č£¬ĖłŅŌĖłµĆĒāĘųµÄÖŹĮæ£ŗ![]() ŃĪĖį
ŃĪĖį![]() “×Ėį£¬
“×Ėį£¬
¹Ź“š°øĪŖ£ŗ![]() £»
£»![]() ”£
ӣ

 ÅąÓÅæŚĖćĢāæØĻµĮŠ“š°ø
ÅąÓÅæŚĖćĢāæØĻµĮŠ“š°ø æŖŠÄæŚĖćĢāæØĻµĮŠ“š°ø
æŖŠÄæŚĖćĢāæØĻµĮŠ“š°ø æŚĖćĢāæØŗÓ±±ÉŁÄź¶łĶƳö°ęÉēĻµĮŠ“š°ø
æŚĖćĢāæØŗÓ±±ÉŁÄź¶łĶƳö°ęÉēĻµĮŠ“š°ø