̀âÄ¿ÄÚÈƯ
ÓĂCH4´ß»¯»¹ÔNOx¿É̉ÔÏû³ưµªÑơ»¯ÎïµÄÎÛȾ¡£
ÀưÈ磺CH4(g)£«4NO2(g)=4NO(g)£«CO2(g)£«2H2O(g)¡¡ ¦¤H1£½£574 kJ¡¤mol£1
CH4(g)£«4NO(g)=2N2(g)£«CO2(g)£«2H2O(g) ¦¤H2£½£1 160 kJ¡¤mol£1
ÈôÔÚ±ê×¼×´¿öÏÂÓĂCH4»¹Ô4.48 L NO2Éú³ÉN2£¬ỘÏÂÁĐ˵·¨ÖĐƠưÈ·µÄÊÇ
(¡¡¡¡)¡£
A£®¸Ă¹ư³̀ÎüÊƠµÄÈÈÁ¿Îª86.7 kJ
B£®´Ë¹ư³̀ÖĐĐè̉ª±ê×¼×´¿öÏÂCH4Æø̀å1.12 L
C£®×ª̉Ƶĵç×ÓÊưΪ0.8NA
D£®̉ÑÖª2NO(g)£«O2(g)=2NO2(g)¡¡¦¤H£½£114 kJ¡¤mol£1£¬ỘCH4µÄȼÉƠÈÈÊÇ802 kJ¡¤mol£1
¡¡C
¡¾½âÎö¡¿¡¡¸ù¾Ư¸Ç˹¶¨Âɽ«Á½¸öÈÈ»¯Ñ§·½³̀ʽÏà¼Ó²¢³ửÔ2¿ÉµĂ£ºCH4(g)£«2NO2(g)=N2(g)£«CO2(g)£«2H2O(g)¡¡¦¤H2£½£867 kJ¡¤mol£1£¬Îª·ÅÈÈ·´Ó¦£¬AÏî´íÎó£»BÏî¼ÆËă´íÎó£¬Ó¦Đè̉ª±ê×¼×´¿öÏÂ2.24 L CH4£»±íʾ¼×ÍéȼÉƠÈȱØĐëÊÇÉú³É̉º̀¬Ë®£¬ÓÉËù¸ø̀ơ¼₫²»ÄܼÆËă£¬¹ÊDÏî´íÎó¡£

 ¿́½ƯÓ¢ÓïÖÜÖÜÁ·ÏµÁĐ´đ°¸
¿́½ƯÓ¢ÓïÖÜÖÜÁ·ÏµÁĐ´đ°¸̉»¶¨Î¶ÈÏ£¬ÔÚ2 LĂܱƠÈƯÆ÷ÖĐ¼ÓÈëÄÉĂ×¼¶Cu2O²¢Í¨Èë0.1 mol H2O(g)£¬·¢Éú·´Ó¦£º2H2O(g)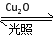 2H2(g)£«O2(g)¡¡¦¤H£½£«484 kJ¡¤mol£1£¬²»Í¬Ê±¼ä²úÉúO2µÄÎïÖʵÄÁ¿¼ûÏÂ±í£º
2H2(g)£«O2(g)¡¡¦¤H£½£«484 kJ¡¤mol£1£¬²»Í¬Ê±¼ä²úÉúO2µÄÎïÖʵÄÁ¿¼ûÏÂ±í£º
ʱ¼ä/min | 20 | 40 | 60 | 80 |
n(O2)/mol | 0.001 0 | 0.001 6 | 0.002 0 | 0.002 0 |
ÏÂÁĐ˵·¨²»ƠưÈ·µÄÊÇ (¡¡¡¡)¡£
A£®Ç°20 minÄÚµÄƽ¾ù·´Ó¦ËÙÂÊv(H2O)£½5.0¡Á10£5 mol¡¤L£1¡¤min£1
B£®´ïµ½Æ½ºâʱ£¬ÖÁÉÙĐè̉ª´ÓÍâ½çÎüÊƠÄÜÁ¿0.968 kJ
C£®Ôö´óc(H2O)£¬¿É̉Ồá¸ßË®µÄ·Ö½âÂÊ
D£®´ß»¯Đ§¹ûÓëCu2O¿ÅÁ£µÄ´óĐ¡ÓĐ¹Ø


