��Ŀ����
17��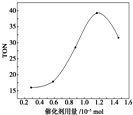 �ڴ��������£����ɼ״���CO2ֱ�Ӻϳ�̼���������CO2+2CH3OH-��CO��OCH3��2+H2O��ij�о�С���������������������£�ͨ���о����������ֱ��ת������TON����Ӱ�������۴����Ĵ�Ч�������㹫ʽΪTON=ת���ļ״������ʵ���/���������ʵ��������ݸ��о�С���ʵ�鼰��������TON��Ӱ��ͼ���ж�����˵������ȷ���ǣ�������
�ڴ��������£����ɼ״���CO2ֱ�Ӻϳ�̼���������CO2+2CH3OH-��CO��OCH3��2+H2O��ij�о�С���������������������£�ͨ���о����������ֱ��ת������TON����Ӱ�������۴����Ĵ�Ч�������㹫ʽΪTON=ת���ļ״������ʵ���/���������ʵ��������ݸ��о�С���ʵ�鼰��������TON��Ӱ��ͼ���ж�����˵������ȷ���ǣ�������| A�� | �ɼ״���CO2ֱ�Ӻϳ�̼����������������ü����õļ״���Ӱ�컷������������CO2ת��Ϊ��Դ������Դѭ�����úͻ����������涼������Ҫ���� | |
| B�� | �ڷ�Ӧ��ϵ�����Ӻ��ʵ���ˮ��������߸÷�Ӧ��TON | |
| C�� | ����������Ϊ1.2��10-5 molʱ���÷�Ӧ��TON�ﵽ��ߵ� | |
| D�� | ��������������1.2��10-5 molʱ�����Ŵ������������ӣ��״���ƽ��ת�������� |
���� A���ڴ��������£����ɼ״���CO2ֱ�Ӻϳ�̼���������DMC����CO2+2CH3OH��CO��OCH3��2+H2O�����Լ��ٶ�����̼���ŷţ�
B�����Ӻ��ʵ���ˮ��������������Ũ�ȼ�С��ƽ�����ƣ�
C����ͼ��ó�����������Ϊ1.2��10-5 molʱ��TON�ı仯��
D�����TON=$\frac{ת���ļ״������ʵ���}{���������ʵ���}$����������жϣ�
��� �⣺�ڴ��������£����ɼ״���CO2ֱ�Ӻϳ�̼���������DMC����CO2+2CH3OH��CO��OCH3��2+H2O��
A�����ݷ�Ӧ��ѧ����ʽ��֪���״��Ͷ�����̼��Ӧ����DMC��ˮ���ɼ״���CO2ֱ�Ӻϳ�DMC���������ü״���Ӱ�컷������������CO2ת��Ϊ��Դ������Դѭ�����úͻ����������涼������Ҫ���壬��A��ȷ��
B���ڷ�Ӧ��ϵ�����Ӻ��ʵ���ˮ��������������Ũ�ȣ�ƽ��������У�����߸÷�Ӧ��TON����B��ȷ��
C����ͼ���֪����������������1.2��10-5molʱ���÷�Ӧ��TON�ﵽ��ߵ㣬��C��ȷ��
D����������������1.2��10-5molʱ�����Ŵ������������ӣ�TOM��С����TOM�ķ�ĸ�������������������Ǽ״���ƽ��ת���ʲ��䣬��D����
��ѡD��
���� ���⿼��ͼ�����������ƽ��Ӱ�����أ��е��Ѷȣ��������������Ϣ�ǽ����Ĺؼ�����Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | �ﵽ��ѧƽ��ʱ������Ӧ���淴Ӧ�����ʶ�Ϊ�� | |
| B�� | �ﵽ��ѧƽ��ʱ��N2��H2��NH3�����ʵ���Ũ�Ȳ��ٱ仯 | |
| C�� | �ﵽ��ѧƽ��ʱ��N2����ȫת��ΪNH3 | |
| D�� | �ﵽ��ѧƽ��ʱ��N2��H2��NH3�����ʵ���Ũ��һ����� |
| A�� | 0.002 0 | B�� | 0.011 | C�� | 0.11 | D�� | 0.22 |
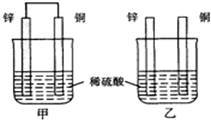
| A�� | ���ձ�����Һ��������Ũ�Ⱦ���С | B�� | ����ͭƬ������������ͭƬ�Ǹ��� | ||
| C�� | ���ձ���ͭƬ����������ݲ��� | D�� | �ס�����Һ������ɫ |
| A�� | ����̼�ᱵ��Ϊ�ڷ���Ӱ��������ΪKsp��BaCO3����Ksp��BaSO4�� | |
| B�� | ���ȱ������ж�����ʱ����û�������ƣ�������̼������Һ���� | |
| C�� | ������c��Ba2+��=1.0��10-5 mol•L-1����Һʱ�������������ж� | |
| D�� | ������0.36 mol•L-1��Na2SO4��Һ���������ж�����ϴθ |
| A�� | 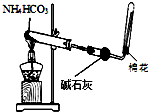 ͼװ��������ȡ�����İ��� | |
| B�� | 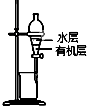 ͼװ��������ȡI2��CCl4��Һ�е�I2 | |
| C�� | 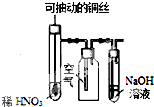 ͼװ����ϡHNO3���ڹ��ƿ�п��ռ�NO���� | |
| D�� | 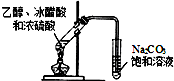 ͼװ�ÿ���ȡ�������������� ���г����ԣ� |
| A�� | 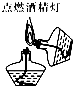 | B�� | 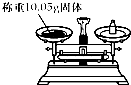 | C�� |  | D�� |  |
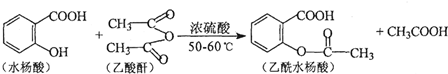
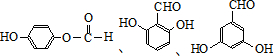 ������һ�֣���
������һ�֣��� ����д��������
����������� ��
��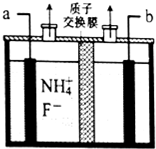 ��ҵ��ͨ����⺬NH4F����ˮ����������NF3���壬����ԭ����ͼ��ʾ����a��Ϊ�������ü��ĵ缫��ӦʽNH4++3F--6e-=NF3+4H+���õ�Ʒ�����������п�����ɷ��������ַ�ֹ������ʴ�ķ�����ԭ�������ڸ��DZ����㷨��
��ҵ��ͨ����⺬NH4F����ˮ����������NF3���壬����ԭ����ͼ��ʾ����a��Ϊ�������ü��ĵ缫��ӦʽNH4++3F--6e-=NF3+4H+���õ�Ʒ�����������п�����ɷ��������ַ�ֹ������ʴ�ķ�����ԭ�������ڸ��DZ����㷨��