��Ŀ����
19��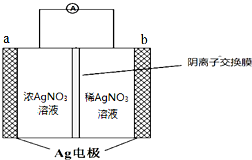 ����һ���ڹ�ҵ���������й㷺��;�Ľ�����
����һ���ڹ�ҵ���������й㷺��;�Ľ�������֪���ٽ�����ˮ�д��������ܽ�ƽ����̣�M?Mx++xe-��������ˮ��Һ��Ҳ��������ƽ����̣�H2?2H++2e-��Ag2S ��Ksp=6.7��10-50�� AgCl��Ksp=1.6��10-10
�ݴ˻ش������й����⣺
��1�����ʲ;߿�ɱ��������ԭ����Ag?Ag++e-��Ag�ܽ��ͷŵ�Ag+���ؽ��������ӣ�ʹϸ�����ڵ����ʱ��ԣ��Ӷ�ɱ�����������ʵ��ķ���ʽ�����ֱ���˵������
��������������ɷ�Ӧ���ɺ�ɫ�������ɫ���壬д���÷�Ӧ�Ļ�ѧ����ʽ2Ag+H2S=Ag2S+H2��
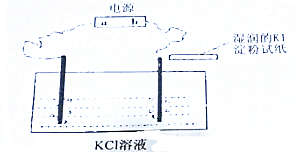
��2������������������Һ��ɵ��ʾ��ͼ���ң�a�缫�ķ�ӦΪAg++e-=Ag��NO3-�ӵ�������Һ�����Ҳ���Һ�ƶ� ������ҡ�����
��3������������������ֽ�ΪAg��NO2��O2����Ӧ������NO2��O2�����ʵ���֮��Ϊ2��1�����������ͨ��ˮ���պ�ʣ������ΪO2��������
��4����֪��Ag+��aq��+2NH3•H2O��aq��?[Ag��NH3��2]+��aq��+2H2O K=1.6��107��д��AgCl���ڰ�ˮ�����ӷ���ʽAgCl+2NH3•H2O��aq��?[Ag��NH3��2]+��aq��+Cl-+2H2O������÷�Ӧ��ƽ�ⳣ��K=2.56��10-3�����Ȼ������ڰ�ˮ�����Һ�еμ�ϡ���ᣬ���ٲ�����ɫ�Ȼ����������μ��������պó�����ȫ��ȡ�ϲ���Һ����pH�����ֳ����ԣ���Ҫԭ����NH4++H2O?NH3•H2O+H+�������ӷ���ʽ��ʾ����
���� ��1��AgΪ�ؽ�������ʹ�����ʱ��ԣ����������H2S��Ӧ����Ag2S��
��2��ԭ���������������Ũ�Ȳ��γ�ԭ��أ������������Ũ��С�ķ����ƶ��������Ϊ�������Ҳ�Ϊ������
��3�����ݵ���ת����ȵ��ص���㣻
��4��AgCl���ڰ�ˮ������Ϸ�Ӧ�����ӷ���ʽΪAgCl+2NH3•H2O��aq��?[Ag��NH3��2]+ ��aq��+Cl-+2H2O��K=$\frac{c[Ag��N{H}_{3}��_{2}]^{+}��c��C{l}^{-}��}{{c}^{2}��N{H}_{3}•{H}_{2}O��}$=$\frac{c[Ag��N{H}_{3}��_{2}]^{+}��c��C{l}^{-}��}{{c}^{2}��N{H}_{3}•{H}_{2}O��}$��$\frac{c��A{g}^{+}��}{c��A{g}^{+}��}$���Դ˼���ƽ�ⳣ������Ӧ��������Σ�ˮ������ԣ�
��� �⣺��1���������Ϣ��֪Ag��ˮ��Һ�з���Ag?Ag++e-������Ag�ܽ��ͷŵ�Ag+ ���ؽ��������ӣ�ʹϸ�����ڵ����ʱ��ԣ��Ӷ�ɱ��������
Ag���������H2S��Ӧ����Ag2S������ʽΪ 2Ag+H2S=Ag2S+H2����
�ʴ�Ϊ��Ag?Ag++e-��Ag�ܽ��ͷŵ�Ag+ ���ؽ��������ӣ�ʹϸ�����ڵ����ʱ��ԣ��Ӷ�ɱ�������� 2Ag+H2S=Ag2S+H2����
��2��ԭ���������������Ũ�Ȳ��γ�ԭ��أ������������Ũ��С�ķ����ƶ��������Ϊ�������Ҳ�Ϊ������
a�缫�ķ�ӦΪAg++e-=Ag��NO3-�ӵ�� �����Һ�����Ҳ���Һ�ƶ�
�ʴ�Ϊ��Ag++e-=Ag�����ң�
��3������������������ֽ�ΪAg��NO2��O2������ʽΪ2AgNO3$\frac{\underline{\;����\;}}{\;}$2Ag+2NO2+O2����Ӧ������NO2��O2�����ʵ���֮��Ϊ2��1��
���������ͨ��ˮ����4NO2+O2+2H2O=4HNO3��������������ʣ��������
�ʴ�Ϊ��2��1��O2����������
��4��AgCl���ڰ�ˮ������Ϸ�Ӧ�����ӷ���ʽΪAgCl+2NH3•H2O��aq��?[Ag��NH3��2]+ ��aq��+Cl-+2H2O��
K=$\frac{c[Ag��N{H}_{3}��_{2}]^{+}��c��C{l}^{-}��}{{c}^{2}��N{H}_{3}•{H}_{2}O��}$=$\frac{c[Ag��N{H}_{3}��_{2}]^{+}��c��C{l}^{-}��}{{c}^{2}��N{H}_{3}•{H}_{2}O��}$��$\frac{c��A{g}^{+}��}{c��A{g}^{+}��}$=1.6��10-10��1.6��107=2.56��10-3��
���Ȼ������ڰ�ˮ�����Һ�еμ�ϡ���ᣬ���ٲ�����ɫ�Ȼ����������μ��������պó�����ȫ��ȡ�ϲ���Һ����pH�����ֳ����ԣ���Ҫԭ����笠�����ˮ������Ե��£�NH4++H2O?NH3•H2O+H+��
�ʴ�Ϊ��AgCl+2NH3•H2O��aq��?[Ag��NH3��2]+ ��aq��+Cl-+2H2O��K=2.56��10-3��NH4++H2O?NH3•H2O+H+��
���� ���⿼���Ϊ�ۺϣ��漰���ܵ���ʵ��ܽ�ƽ�⡢ԭ��ء�ƽ�ⳣ���ļ����֪ʶ��Ϊ�߿��������ͣ�������ѧ���ķ��������������Ŀ��飬ע����յ缫����ʽ����д�Լ�ƽ�ⳣ���ļ��㣮

 ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�| A�� | ���ʹ��Na2CO3����Һ�У�2c��Na+��=c��CO32-��+c��HCO3-��+c��H2CO3�� | |
| B�� | 1mol/L�ģ�NH4��2SO4��Һ�У�c��NH4+����c��SO42-����c��H+����c��OH-�� | |
| C�� | 0.10mol/L��������Һ�У�c��Na+��+c��H+��=c��CH3COO-��+c��OH-�� | |
| D�� | �������pH=3������ʹ����к��������Ƶ����ʵ�����ͬ |
 �״���һ����Ҫ�Ļ���ԭ�ϣ�
�״���һ����Ҫ�Ļ���ԭ�ϣ���1����֪����2CH3OH��l��+3O2��g��=2CO2��g��+4H2O��g����H=-1275.6kJ•mol-1
��H2O��l��=H2O��g����H=+44.0kJ•mol-1
д����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽCH3OH��l��+$\frac{3}{2}$O2��g��=CO2��g��+2H2O��l����H=-725.8kJ/mol��
��2���״���ˮ�����������ɻ�������Դ�����й㷺��Ӧ��ǰ�����䷴ӦΪ��
CH3OH ��g��+H2O ��g��?CO2��g��+3H2��g����H=-72.0kJ/mol
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪK=$\frac{c��C{O}_{2}��{c}^{3}��{H}_{2}��}{c��C{H}_{3}OH��c��{H}_{2}O��}$��
�����д�ʩ����ʹƽ��ʱ$\frac{n��C{H}_{3}OH��}{n��C{O}_{2}��}$��С���ǣ�˫ѡ��CD��
A��������� B�����ݳ���He��g����ʹ��ϵѹǿ����
C����H2��g������ϵ�з��� D�������ٳ���1molH2O��g��
��3���״����������ɼ��ᣬ�ڳ�������0.1000mol/L NaOH��Һ�ζ�20.00mL 0.1000mol/L ������Һ�����У������Һ��pH=7ʱ�������ĵ�V��NaOH����
�������������=���� 20.00mL��
��4�����ü״�ȼ�����Ϊȼ�ϵ�أ���ͼ��ʾ�����缫��ӦʽΪCH3OH-6e-+8OH-=CO32-+6H2O��
��5���ϳɼ״�����Ҫ��ӦΪ��CO��g��+2H2��g��?CH3OH��g����H=-90.8kJ/molԭ�����ļӹ������г�������һЩCO2��Ϊ���о��¶ȼ�CO2�����Ը÷�Ӧ��Ӱ�죬��CO2��CO��H2�Ļ������Ϊԭ����һ�������½���ʵ�飮ʵ�����ݼ��±���
| CO2%-CO%-H2% ����������� | 0-30-70 | 2-28-70 | 4-26-70 | 8-22-70 | ||||||||
| ��Ӧ�¶�/�� | 225 | 235 | 250 | 225 | 235 | 250 | 225 | 235 | 250 | 225 | 235 | 250 |
| ����CH3OH��̼ת���ʣ�%�� | 4.9 | 8.8 | 11.0 | 36.5 | 50.7 | 68.3 | 19.0 | 33.1 | 56.5 | 17.7 | 33.4 | 54.4 |
����һ����һ�������£���Ӧ�¶�Խ�ߣ�����CH3OH��̼ת����Խ�ߣ�
���۶���ԭ����������CO2������������ɼ״���̼ת���ʣ�CO2�����������ɼ״���̼ת�����ֽ��ͣ�
| A�� | ��ʹ�����Ժ�ɫ����Һ�д������ڣ�Mg2+��Na+��Cl-��F- | |
| B�� | ��״���£�46gNO2��N2O4��������к���ԭ�Ӹ���Ϊ3NA | |
| C�� | 1L0.5mol•L-1 CuSO4��Һ�к���0.5NA��Cu2+ | |
| D�� | Ũ�Ⱦ�Ϊ0.1 mol/L�İ�ˮ�����ᡢ��ˮ�������c��H+���������ˮ |
| A�� | C3H7Cl | B�� | C3H6Cl2 | C�� | C3H5Cl3 | D�� | C3HCl7 |

 ��
��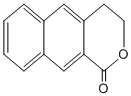 ��һ����Ҫ��ҩ��ϳ��м��壬������Ŀ������Ϣ��
��һ����Ҫ��ҩ��ϳ��м��壬������Ŀ������Ϣ��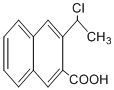 Ϊԭ���Ʊ��û�����ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�
Ϊԭ���Ʊ��û�����ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�