题目内容
四种短周期元素A、B、C、D的性质或结构信息如下。

信息① 原子半径大小:A>B>C>D
信息② 四种元素之间形成的某三种分子的比例模型及部分性质:
甲:是地球上最常见的物质之一,是包括人类在内所有生命生存的重要资源,也是生物体最重要的组成部分。
乙:无色,无味而易燃,是21世纪的主要能源。
丙:有强氧化性,可以用于消毒杀菌。请根据上述信息回答下列问题。
(1)丙 (写化学式)写出其分子的的电子式 。
(2)写出A原子最外层电子排布式 。将该元素的单质溶于水,形成的平衡体系中所有离子的浓度按由大到小排序:
(3)B形成的单质晶体可能为 。
A.离子晶体 B.分子晶体 C.原子晶体 D.金属晶体
(4)C的同主族元素的单质及其化合物的性质存在着相似性和递变性。下列有关说法正确的是____
A.其气态氢化物的稳定性按H2O、H2S、H2Se、H2Te的顺序依次减弱
B.其氢化物中的键长按O—H、S—H、Se—H、Te—H的顺序依次减小
C.其氢化物的沸点按H2O、H2S、H2Se、H2Te的顺序依次增强
D.其阴离子的还原性按O2–、S2–、Se2–、Te2–的顺序依次增强
(5)与氩原子质子数和电子数均相同的微粒有HCl、H2S、PH3、SiH4,以及还有_______ 、___________等(例举两种即可)

信息① 原子半径大小:A>B>C>D
信息② 四种元素之间形成的某三种分子的比例模型及部分性质:
甲:是地球上最常见的物质之一,是包括人类在内所有生命生存的重要资源,也是生物体最重要的组成部分。
乙:无色,无味而易燃,是21世纪的主要能源。
丙:有强氧化性,可以用于消毒杀菌。请根据上述信息回答下列问题。
(1)丙 (写化学式)写出其分子的的电子式 。
(2)写出A原子最外层电子排布式 。将该元素的单质溶于水,形成的平衡体系中所有离子的浓度按由大到小排序:
(3)B形成的单质晶体可能为 。
A.离子晶体 B.分子晶体 C.原子晶体 D.金属晶体
(4)C的同主族元素的单质及其化合物的性质存在着相似性和递变性。下列有关说法正确的是____
A.其气态氢化物的稳定性按H2O、H2S、H2Se、H2Te的顺序依次减弱
B.其氢化物中的键长按O—H、S—H、Se—H、Te—H的顺序依次减小
C.其氢化物的沸点按H2O、H2S、H2Se、H2Te的顺序依次增强
D.其阴离子的还原性按O2–、S2–、Se2–、Te2–的顺序依次增强
(5)与氩原子质子数和电子数均相同的微粒有HCl、H2S、PH3、SiH4,以及还有_______ 、___________等(例举两种即可)
(1)HClO(1分)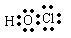 l(1分)。
l(1分)。
(2)3S23P5 (1分) [H+]>[Cl-]>[ClO-]>[OH-] (1分)
(3)BC(1分)
(4)AD(1分)
(5)F2 、H2O2、N2H4、C2H6、CH3OH (2分)
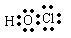 l(1分)。
l(1分)。(2)3S23P5 (1分) [H+]>[Cl-]>[ClO-]>[OH-] (1分)
(3)BC(1分)
(4)AD(1分)
(5)F2 、H2O2、N2H4、C2H6、CH3OH (2分)
试题分析:根据信息确定甲为水,乙为甲烷,丙为次氯酸;A、B、C、D分别为Cl、C、O、H四种元素。(1)次氯酸分子中氧原子分别与氢原子和氯原子形成1对共用电子对;
(2)氯气溶于水部分与水反应,Cl2+H2O
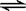 HCl+HClO,HCl为强电解质完全电离,次氯酸是弱电解质,部分水解HClO
HCl+HClO,HCl为强电解质完全电离,次氯酸是弱电解质,部分水解HClO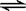 H++ClO-,所以溶液中离子浓度为 [H+]>[Cl-]>[ClO-]>[OH-] ;
H++ClO-,所以溶液中离子浓度为 [H+]>[Cl-]>[ClO-]>[OH-] ;(3)碳元素可以形成多种单质,金刚石为原子晶体,C60为分子晶体,石墨为混合晶体等;
(4)氧族元素从上到下半径依次增大,非金属性减弱,金属性增强。A、气态氢化物稳定性与元素的非金属性一致,正确;B、键长与原子半径一致,错误;C、水中含有分子间氢键,溶沸点较高,错误;D、简单阴离子的还原性与其原子的非金属性相反,正确;
(5)18电子微粒有F2 、H2O2、N2H4、C2H6、CH3OH ,其中前两个重要。

练习册系列答案
相关题目
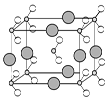

 键与
键与 键的个数比为 。
键的个数比为 。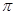 键有 mol。
键有 mol。

