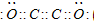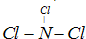��Ŀ����
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E��ͬ��Ԫ�أ���D�����������Ǵ�����������3����A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬����ش���������(���ʱ�û�ѧ��������)��
(1)BC���ĵ���ʽΪ ��Ԫ��F�����ڱ��е�λ��Ϊ ��
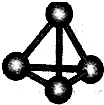
 (2)��������������ѧ��FuNvio Cacace���˻���˼��������о������C4���ӡ�C4���ӽṹ����ͼ��ʾ������������Ϣ�����ݣ�����˵����ȷ����
��
(2)��������������ѧ��FuNvio Cacace���˻���˼��������о������C4���ӡ�C4���ӽṹ����ͼ��ʾ������������Ϣ�����ݣ�����˵����ȷ����
��
A��C4����һ�����͵Ļ����� B��C4��C2��Ϊͬ��������
C��C4�ȶ��Ա�P4(����)�� D��C4��C2��Ϊͬλ��
(3)A��C����Ԫ�ؿ��γɶ��ֻ�������ӣ�����CA3���ӵķ��ӵĿռ乹��Ϊ
������ ��C2A4���ӵĽṹʽΪ ��
(4)Ϊ�˳�ȥ������A2ED4ϡ��Һ�л��е�A2ED3��������A2D2Ϊ��������������Ӧ�����ӷ���ʽΪ ��
(5)E��F�γɵĻ�����E2F2����ҵ������Ҫ��;����ˮ��ˮ�⣬��ռ�ṹ��A2D2��Ϊ���ơ��Դ�����˵����ȷ����
A��E2F2�ĽṹʽΪ��F��E��E��F B��E2F2��һ�������£��������Ի�ԭ��
C��E2F2�е�����Eԭ�ӵĻ��ϼ۷ֱ�Ϊ0�ۺ�+2��
D��E2F2��H2O��Ӧ�Ļ�ѧ����ʽ����Ϊ��2E2F2+2H2O=EO2��+ 3E��+4HF
(6)B��D���γ����ֳ������������һ�ֻ�����BD2�� ����(����ԡ��Ǽ��ԡ�)����1000 mL 0.1 mol/L�� Ca(OH)2���ʵ�ˮ��Һ����ͨ��BD2����ֽ��裬��Һ���������������������仯������ͼ�л�������������ͨ��BD2�����ʵ����ı仯���ߡ�
 |
(��14��)(1) (2��) �����ڢ�A��(1��)
(2)B(1��)
(3)������(1��) 107O18��(1��) (2��)
(4)H2SO3+H2O2=2H++SO42��+H2O(1��) (5)ABD(2��)
(6)�Ǽ���(1��) ͼ��2��
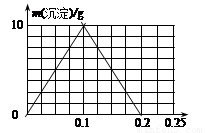
��������

 ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��