��Ŀ����
��֪��Cu(OH)2�Ƕ�Ԫ��������ᣨH3PO3���Ƕ�Ԫ���ᣬ��NaOH��Һ��Ӧ������Na2HPO3��
��1����ͭ����Һ��Cu2������ˮ�ⷴӦ�����ӷ���ʽΪ____���÷�Ӧ��ƽ�ⳣ��Ϊ____������֪��25��ʱ��Ksp[Cu(OH)2]��2.0��10��20mol3/L3��
��2������H3PO3�����ʿ��Ʋ�Na2HPO3ϡ��Һ��pH______7��������������������������£���10mL0.01mol/L H3PO3��Һ�еμ�10ml0.02mol/LNaOH��Һ����Һ�и�������Ũ���ɴ�С��˳����_________��
��3�����Na2HPO3��Һ�ɵõ������ᣬװ����ͼ��˵������Ĥֻ����������ͨ������Ĥֻ����������ͨ����

�������ĵ缫��ӦʽΪ____________________��
�ڲ�Ʒ���з�Ӧ�����ӷ���ʽΪ____________��
��1����ͭ����Һ��Cu2������ˮ�ⷴӦ�����ӷ���ʽΪ____���÷�Ӧ��ƽ�ⳣ��Ϊ____������֪��25��ʱ��Ksp[Cu(OH)2]��2.0��10��20mol3/L3��
��2������H3PO3�����ʿ��Ʋ�Na2HPO3ϡ��Һ��pH______7��������������������������£���10mL0.01mol/L H3PO3��Һ�еμ�10ml0.02mol/LNaOH��Һ����Һ�и�������Ũ���ɴ�С��˳����_________��
��3�����Na2HPO3��Һ�ɵõ������ᣬװ����ͼ��˵������Ĥֻ����������ͨ������Ĥֻ����������ͨ����

�������ĵ缫��ӦʽΪ____________________��
�ڲ�Ʒ���з�Ӧ�����ӷ���ʽΪ____________��
��13�֣���1��Cu2+��2H2O Cu(OH)2��2H+��2�֣���5��10��9��2�֣�
Cu(OH)2��2H+��2�֣���5��10��9��2�֣�
��2������2�֣���c(Na+)��c(HPO32��)��c(OH��)��c(H2PO3��)��c(H+)��2�֣�
��3����4OH�D�D4e����2H2O��O2����2�֣�
��HPO32����2H����H3PO3��2�֣���HPO32����H����H2PO3����H2PO3����H����H3PO3����1�֣�
 Cu(OH)2��2H+��2�֣���5��10��9��2�֣�
Cu(OH)2��2H+��2�֣���5��10��9��2�֣���2������2�֣���c(Na+)��c(HPO32��)��c(OH��)��c(H2PO3��)��c(H+)��2�֣�
��3����4OH�D�D4e����2H2O��O2����2�֣�
��HPO32����2H����H3PO3��2�֣���HPO32����H����H2PO3����H2PO3����H����H3PO3����1�֣�
�����������1��������ͭ���������ͭ���ӿ���ˮ�⣬��Һ�����ԣ���ˮ�ⷴӦ�����ӷ���ʽΪCu2+��2H2O
 Cu(OH)2��2H+����ѧƽ�ⳣ������һ�������£������淴Ӧ�ﵽƽ��״̬ʱ��������Ũ�ȵ���֮���ͷ�Ӧ��Ũ�ȵ���֮���ı�ֵ�����Ը÷�Ӧ��ƽ�ⳣ��K��
Cu(OH)2��2H+����ѧƽ�ⳣ������һ�������£������淴Ӧ�ﵽƽ��״̬ʱ��������Ũ�ȵ���֮���ͷ�Ӧ��Ũ�ȵ���֮���ı�ֵ�����Ը÷�Ӧ��ƽ�ⳣ��K��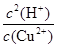 ��
��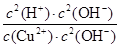 =
=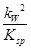 ��
��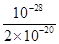 ��5��10��9��
��5��10��9����2��H3PO3�����ᣬNa2HPO3��ǿ�������Σ�����HPO32��ˮ�⣬��ˮ��Һ�ʼ��ԣ���pH��7����10mL0.01mol/LH3PO3��Һ�еμ�10ml 0.02mol/LNaOH��Һ����ǡ�÷�Ӧ����Na2HPO3����Һˮ���Լ��ԣ�������Һ������Ũ�ȴ�СΪc(Na+)��c(HPO32��)��c(OH��)��c(H2PO3��)��c(H+)��
��3���ٵ�������ʧȥ���ӣ�����������Ӧ�������õ����ӷ�����ԭ��Ӧ�����Ը���װ��ͼ��֪������������������ʧ���ӷ���������Ӧ���缫��ӦʽΪ4OH�D�D4e����2H2O��O2����
��������Ĥֻ����������ͨ������Ĥֻ����������ͨ�������Բ�Ʒ����HPO32���������ӽ�����������ᣬ��Ӧ���ӷ���ʽΪHPO32����2H����H3PO3����HPO32����H����H2PO3����H2PO3����H����H3PO3��

��ϰ��ϵ�д�
�����Ŀ