��Ŀ����
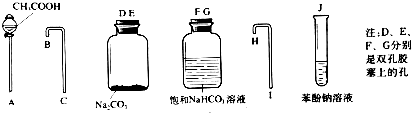
14������ʵ�鷽���У��ܲⶨNa2CO3��NaCl�������NaCl�����������ǣ�������| A�� | ȡa�˻����������Ũ�����ַ�Ӧ��ͨ���ű���NaHCO3��Һ�����������b������״���� | |
| B�� | ȡa�˻������������������Һ��ַ�Ӧ�����ˡ�ϴ�ӡ���ɣ���b�˹��� | |
| C�� | ȡa�˻����������ϡ�����ַ�Ӧ���ݳ������ü�ʯ�����գ�����b�� | |
| D�� | ȡa�˻����������Ba��OH��2��Һ��ַ�Ӧ�����ˣ�������b�˹��� |
���� A��̼���������ᷴӦ��������ӷ���������̼�����Ʒ�Ӧ���ɶ�����̼��
B��bg����Ϊ̼������AgCl��agΪNa2CO3��NaCl��
C��̼���������ᷴӦ���ɶ�����̼����ʯ�����յ�����bgΪ������̼��ˮ��������
D��̼����������������Ӧ����̼�ᱵ���������ˡ�ϴ�ӡ���ɺ�Ĺ�������Ϊ̼�ᱵ��������
��� �⣺A��̼���������ᷴӦ��������ӷ���������̼�����Ʒ�Ӧ���ɶ�����̼����bL���岻��ȫ��Դ��̼���ƣ����ܲⶨ����A����
B��bg����Ϊ̼������AgCl��agΪNa2CO3��NaCl����Na2CO3��NaCl�������ֱ�Ϊx��y����������ϵ�õ�������$\left\{\begin{array}{l}{x+y=a}\\{\frac{x}{106}��266+\frac{y}{58.5}��143.5=b}\end{array}\right.$���Դ˿ɼ���NaCl��������������������B��ȷ��
C��̼���������ᷴӦ���ɶ�����̼����ʯ�����յ�����bgΪ������̼��ˮ�����������ܼ���̼���Ƶ����������ܲⶨNaCl��������������C����
D��̼����������������Ӧ����̼�ᱵ���������ˡ�ϴ�ӡ���ɺ�Ĺ�������Ϊ̼�ᱵ������������û��ϴ�ӡ���ɣ���b�˹���������һ��Ϊ̼�ᱵ���������ܲⶨ����D����
��ѡB��
���� ���⿼�黯ѧʵ�鷽�������ۼ����ʺ����IJⶨ��Ϊ��Ƶ���㣬���շ����ķ�Ӧ�������������ж�Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬��Ŀ�ѶȲ���

| A�� | 2��2-�������� | B�� | 1��2-���ȶ��� | C�� | 2-��-2-��ϩ | D�� | 2-��-3-��Ȳ |
| A�� | 10��ʱ��HCl��H2O | B�� | 400Kʱ��ˮ��CO2 | ||
| C�� | 20��ʱ�������Ϳ��� | D�� | �����µ�H2��N2 |
| ��� | ʵ������ | ʵ��Ŀ�Ļ�ʵ����� |
| �� | ����һ��ǿ�����䷯��Һ�����������ġ�ͨ·���� | ˵������һ��������ˮ�� |
| �� | ��bmL0.1mol/LAgNO3��Һ�еμ�1mL0.1mol/LNaCl��Һ���а�ɫ�������ɣ��������еμ�1mL0.1mol/LKI��Һ���л�ɫ�������ɣ� | ��֤AgCl���ܽ�ȱ�AgI�� |
| �� | ��KI��Һ�е���CuSO4��Һ���а�ɫ�������ɣ���������������ټ���������̼��������̼�����ɫ | ˵����ɫ��������ΪCuI |
| �� | �����£��ֱ���2֧�Թ��м�����ͬ�������ͬŨ�ȵ�Na2S2O3��Һ���ٷֱ����������ͬŨ�ȵ�ϡ���� | �о�Ũ�ȶԷ�Ӧ���ʵ�Ӱ�� |
| �� | ������Ͷ�뵽ʢ��ϡHNO3���Թ��У���ַ�Ӧ����뼸��KSCN��Һ���������� | ˵��HNO3��Fe������Fe2+ |
| A�� | �٢� | B�� | �ۢ� | C�� | �ۢ� | D�� | �ڢ� |
| A�� | 90��ʱ����ˮ��pH����7 | |
| B�� | ����FeCl3��Һ��Ϊ������ˮ�⣬����ϡ���� | |
| C�� | ��֪һ��c ��H+��=1��10-3mol/L�����һ��c ��OH-��=1��10-3mol/L����Һ�Ե������Ϻ���Һ�����ԣ���ԭ����Ũ�������ϡ�Ӧ���� | |
| D�� | ��Na2S��Һ�У�����Ũ�ȵĹ�ϵ�ǣ�c ��Na+����c ��S2-����c ��HS-����c ��OH-����c ��H+�� |
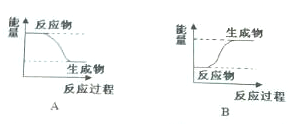
 ij��ȤС����С����ý������ᷴӦ����ʵ�飬��5.4g����ƬͶ��500mL 0.5mol•L-1��������Һ�У���ͼΪ��Ӧ�������������뷴Ӧʱ��Ĺ�ϵͼ��
ij��ȤС����С����ý������ᷴӦ����ʵ�飬��5.4g����ƬͶ��500mL 0.5mol•L-1��������Һ�У���ͼΪ��Ӧ�������������뷴Ӧʱ��Ĺ�ϵͼ��