��Ŀ����
����Ŀ��ʵ������50mL0.50mol��L-1���ᡢ50mL0.50mol��L-1NaOH��Һ����ͼ��ʾװ�ý��вⶨ�к��ȵ�ʵ�飬�õ����е����ݣ�
ʵ����� | ��ʼ�¶� | ��ֹ�¶� | |
���� | NaOH��Һ | ||
1 | 20.2 | 20.3 | 23.7 |
2 | 20.3 | 20.5 | 23.8 |
3 | 21.5 | 21.6 | 24.9 |
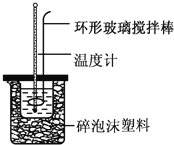
��1��������ͭ˿��������滷�β����������������_______
��2�������ϱ����������ݽ��м��㣬���ʵ���õ��к�����H=__________�������NaOH��Һ���ܶȰ�1gcm-3���㣬��Ӧ������Һ�ı�����C��4.18J��g�棩-1���㣬�������С�����һλ����
��3������NaOH��Һ��Ϊ��ͬ�������ͬŨ�ȵİ�ˮ����ʵ���в�õ����к�����Ϊ��H1������H1����H�Ĺ�ϵΪ��H1________��H����������������С�����������������������60mL0.5mol��L-1��������50mL 0.55mol��L-1��NaOH ��Һ���з�Ӧ��������ʵ����ȣ����ų�������_______�������������������������������к���___��������������������������
��4����ijͬѧ��������װ����ʵ�飬��Щ�������淶����ɲ���к��ȵ���ֵƫ�ͣ�����������ܵ�ԭ����___������ĸ��ţ���
A ����������¶Ⱥ��¶ȼ�û����ˮ��ϴ�ɾ�
B ����Ͳ�е�����������Һ����С�ձ�ʱ�����ٻ�
C ����ʵ��ĵ������½ϸ�
D ��50mL0.55mol/L����������Һȡ����50mL0.55mol/L�İ�ˮ
E ����ȡ����ʱ���Ӽ���
F ���ձ��ĸǰ��м�С��̫��
���𰸡�Cu���ȿ죬������ʧ�� -56.8kJ��mol-1 ���� ����� ��� ABDF
��������
��1���������ȿ죬������ʧ�ࣻ
��2���ȸ��ݱ��вⶨ���ݼ�������Һ��Ӧǰ���ƽ���¶Ȳ�ٸ���Q=m��c����T�������Ӧ�ų���������Ȼ����������1molˮ�ų����������Ϳ��Եõ��к��ȣ�
��3����ˮΪ������ʣ����������Ҫ������������Ӧ�ų����������Ӧ��һЩ����Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
��4������ʵ��ɹ��Ĺؼ��DZ��£����װ��������ɢʧ����ᵼ�½��ƫ�ͣ�����ʵ�����õ����Լ��Լ�ʵ�����֪ʶ���жϡ�
��1�����ܽ����β����������Ϊͭ˿���������Ϊͭ˿��������ȵ������壻
��Ϊ��Cu���ȿ죬������ʧ��
��2����1��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ20.25������Ӧǰ���¶Ȳ�Ϊ��3.45����
��2��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ20.40������Ӧǰ���¶Ȳ�Ϊ��3.40����
��3��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ21.55������Ӧǰ���¶Ȳ�Ϊ��3.35����
�����¶Ȳ��ƽ��ֵΪ3.40����
50mL0.50molL1���ᡢ50mL0.55molL1NaOH��Һ������m=100mL��1g/mL=100g��c=4.18J/(g��)�����빫ʽQ=cm��T������0.025mol��ˮ�ų�����Q=4.18J/(g��)��100g��3.40��=1421.2J=1.4212kJ��������0.025mol��ˮ�ų�����1.4212kJ����������1mol��ˮ�ų�����Ϊ![]() =56.8kJ������ʵ���õ��к��ȡ�H=56.8kJ/mol��
=56.8kJ������ʵ���õ��к��ȡ�H=56.8kJ/mol��
�ʴ�Ϊ��56.8kJ/mol��
��3����ˮΪ������ʣ����������Ҫ������������Ӧ�ų����������Ӧ��һЩ���������������Qƫ�ͣ����¡�H1���ڡ�H����Ӧ�ų����������������Լ�������Ķ����йأ�����60mL0.50mol/L�����50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ��к�����ȣ�
�ʴ�Ϊ�����ڣ�����ȣ���ȣ�
��4��A������������¶Ⱥ��¶ȼ�û����ˮ��ϴ�ɾ����ڲ����¶�ʱ���ᷢ����ͼ���кͣ��¶ȼ�ʾ���仯ֵ��С�����Ե���ʵ�����к��ȵ���ֵƫС����A��ȷ��
B������Ͳ�е�����������Һ����С�ձ�ʱ�����ٻ����ᵼ��һ����������ɢʧ��ʵ�����к��ȵ���ֵƫС����B��ȷ��
C������ʵ������ºͷ�Ӧ�ȵ�����֮���أ���C����
D����50mL0.55mol/L����������Һȡ����50mL0.55mol/L�İ�ˮ�����ڰ�ˮ������������Ҫ���ȣ�����ʵ�����к��ȵ���ֵƫС����D��ȷ��
E������ȡ����ʱ���Ӽ�������ʹ��ʵ����ȡ���������Ҫ�������������������Ա�֤��ȫ��Ӧ������ʵ�����к��ȵ���ֵƫ�ߣ���E����
F�����ձ��ĸǰ��м�С��̫�ᵼ��һ��������ɢʧ������ʵ�����к��ȵ���ֵƫС����F��ȷ��
��ѡ��ABDF��