题目内容
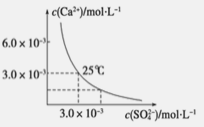
【题目】已知25 ℃时,CaSO4在水中的沉淀溶解平衡曲线如图所示,该条件下向100 mL的CaSO4饱和溶液中,加入200 mL 0.03 mol·L -1 的Na2SO4溶液,针对此过程的下列叙述正确的是(忽略混合过程中的体积变化)
A.溶液中析出CaSO4沉淀,最终溶液中c(SO42-)较原来大
B 溶液中析出CaSO4沉淀,溶液中c(Ca2+ )、c(SO 42-)都变小
C.溶液中无沉淀析出,溶液中c(Ca2+ )、c(SO42-)都变小
D.溶液中无沉淀析出,但最终溶液中c(SO42-)较原来大
【答案】A
【解析】
试题分析:由图示可知,在该条件下,CaSO4饱和溶液中,c(Ca2+)=c(SO42-)=3.0×10-3mol/L,Ksp(CaSO4)=9.0×10-6。当向100 mL该条件下的CaSO4饱和溶液中加入200 mL 0.03mol/L的Na2SO4溶液后,混合液中c(Ca2+)=![]() ,c(SO42-)=
,c(SO42-)=![]() ,溶液中c(Ca2+)c( SO42- )=2.1×10-5>Ksp(CaSO4)=9.0×10-6,所以混合液中有沉淀析出,最终溶液中硫酸根离子浓度增大;答案选A。
,溶液中c(Ca2+)c( SO42- )=2.1×10-5>Ksp(CaSO4)=9.0×10-6,所以混合液中有沉淀析出,最终溶液中硫酸根离子浓度增大;答案选A。

练习册系列答案
 数学奥赛暑假天天练南京大学出版社系列答案
数学奥赛暑假天天练南京大学出版社系列答案
相关题目