题目内容
20.某研究小组模拟工业上以黄铁矿为原料制备硫酸的第一步反应为:4FeS2+11O2高温_2Fe2O3+8SO2
进行如图1实验,并测定该样品中FeS2样品的纯度(假设其它杂质不参与反应).
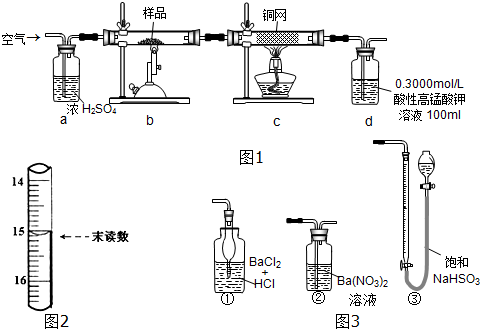
实验步骤:称取研细的样品4.000g放入上图b装置中,然后在空气中进行煅烧.为测定未反应高锰酸钾的量(假设其溶液体积保持不变),实验完成后取出d中溶液10mL置于锥形瓶里,用0.1000mol/L草酸(H2C2O4)标准溶液进行滴定.
【提示:装置c中铜网作用是除去SO2气体混有的氧气,以保证在装置d中只有一种气体参与反应】
(已知:2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2↑+2MnSO4+8H2O)
请回答下列问题:
(1)称量样品质量能否用托盘天平不能(填“能”或“不能”);
(2)装置a的作用是干燥空气(或干燥或除去水蒸气均可),观察气体流速;
(3)上述反应结束后,仍需通一段时间的空气,其目的是促进装置中的二氧化硫气体全部吸收;
(4)写出d中发生反应的离子方程式:5SO2+2MnO4-+2H2O═5SO42-+2Mn2++4H+;
(5)滴定时,已知滴定管初读数为0.10ml,末读数如图2所示,消耗草酸溶液的体积为15.00ml;
(6)该样品中FeS2的纯度为0.90;(用小数表示,保留两位小数)
(7)若用图3装置替代上述实验装置d,同样可以达到实验目的是②.(填编号)
分析 (1)称取研细的样品4.000g,由质量的精确度可知不能用托盘天平来称量;
(2)装置a为除去空气中的水蒸气避免影响测定结果,同时可以观察气体流速控制反应进行的速率;
(3)继续通入空气目的是把装置中的二氧化硫气体全部赶出完全吸收;
(4)草酸被高锰酸钾溶液氧化为二氧化碳,高锰酸钾被还原为锰离子;
(5)依据滴定管的结构,利用开始和结束的体积差值计算得到消耗草酸溶液的体积;
(6)依据灼烧反应和滴定反应的定量关系计算硫化亚铁的质量分数;
(7)装置d是用于吸收并测定产物中二氧化硫的含量,根据二氧化硫的化学性质进行判断.
解答 解:(1)称取研细的样品4.000g,由质量的精确度可知不能用托盘天平来称量,托盘天平的精确度为0.1g,
故答案为:不能;
(2)分析装置图可知为准确测定样品中FeS2样品的纯度,利用空气中的氧气氧化硫化亚铁反应生成二氧化硫被高锰酸钾溶液吸收后,用草酸滴定计算,过程中空气中的水蒸气会干扰测定,需要通过浓硫酸除去,所以装置 a的作用是干燥或除去水蒸气,同时根据气泡冒出观察气体流速,
故答案为:干燥空气,观察气体流速;
(3)继续通入空气目的是把装置中的二氧化硫气体全部赶出完全吸收,减少误差,
故答案为:促进装置中的二氧化硫气体全部吸收;
(4)草酸被高锰酸钾溶液氧化为二氧化碳,高锰酸钾被还原为锰离子,反应的离子方程式为:2MnO4-+5H2C2O4+6H+=2Mn2++10CO2↑+8H2O,
故答案为:2MnO4-+5H2C2O4+6H+=2Mn2++10CO2↑+8H2O;
(5)依据滴定管的结构,利用开始和结束的体积差值计算得到消耗草酸溶液的体积为:15.10mL-0.10mL=15.00mL,
故答案为:15.00;
(6)依据反应的离子方程式计算与二氧化硫反应的高锰酸钾物质的量,得到二氧化硫的物质的量,结合元素守恒计算硫化亚铁的质量分数,结合反应的离子方程式计算得到:2MnO4-+5H2C2O4+6H+=2Mn2++10CO2↑+8H2O,100mL溶液中剩余高锰酸钾物质的量为:25×0.01500L×0.1000mol/L×10=0.006mol,与二氧化硫反应的高锰酸钾物质的量为:0.3000mol/L×0.1000L-0.006mol=0.024mol,结合反应5SO2+2KMnO4+2H2O═K2SO4+2MnSO4+2H2SO4可得:
5FeS2~10SO2~4KMnO4
5 4
n(FeS2) 0.024mol
n(FeS2)=0.03mol
样品中FeS2的纯度为:0.03mol×120g/mol4.000g=0.90,
故答案为:0.90;
(7)图3中,只有②能够与二氧化硫反应生成硫酸钡沉淀,可以替代装置d;而①无法吸收二氧化硫,③中空气不溶于水,干扰了二氧化硫的测定,
故答案为:②.
点评 本题考查了物质组成的实验探究方法分析,题目难度中等,理解反应原理和滴定实验的计算是解题关键,试题充分考查学生的分析、理解能力及化学实验能力.

| A. | 常温下0.4 mol/L HB溶液与0.2 mol/L NaOH溶液等体积混合后溶液的pH=3,则混合溶液中离子浓度的大小顺序为:c (Na+)>c (B-)>c (H+)>c (OH-) | |
| B. | 常温时,pH均为2的CH3COOH溶液和HCl溶液,pH均为12的氨水和NaOH溶液,四种溶液中由水电离的c(H+)相等 | |
| C. | 常温下0.1 mol/L的下列溶液 ①NH4Al(SO4)2、②NH4Cl、③NH3•H2O、④CH3COONH4中c (NH+4)由大到小的顺序是:②>①>④>③ | |
| D. | 0.1 mol/L NaHB溶液中其pH为4:c(HB-)>c(H2B)>c(B2−) |
| A. | “纳米材料”是指微粒直径为几纳米到几十纳米的材料,故纳米材料是胶体 | |
| B. | 用丁达尔效应可区分胶体和溶液 | |
| C. | 分散剂一定是液体 | |
| D. | 将饱和FeCl3溶液滴入NaOH浓溶液中,可制得Fe(OH)3胶体 |
| A. | 上述反应中氧化产物只有N2 | |
| B. | 经测定,NaCN的水溶液呈碱性,说明CN-能促进水的电离 | |
| C. | 若上述反应生成0.4 mol CO2,则溶液中阴离子增加的物质的量为2mol | |
| D. | 现取1 L含CN-1.02 mg/L的废水,至少需要4.0×10-5mol 氯气处理后才符合排放标准 |
| A. | NaCl | B. | Cu | C. | NaOH | D. | CH3CH2OH |
| A. | 吸烟有害健康 | |
| B. | 尼古丁中C、H、N三种元素的质量比为5:7:1 | |
| C. | CO比O2更易同血红蛋白结合,会导致人体缺氧 | |
| D. | 尼古丁中氮元素的质量分数约为17.3% |
| A. |  可用于NaCl与NH4Cl混合物的分离 | |
| B. |  用于去除Fe(OH)3胶体中含有的可溶性物质 | |
| C. |  橡皮管起到平衡气压、使液体顺利流下的作用 | |
| D. |  若该仪器为酸式滴定管,则表示其内液体体积为5.00mL |
| A. | 标准状况下,22.4L SO3含有的原子数为4NA | |
| B. | 78 g过氧化钠中含有的离子数为4NA | |
| C. | 过氧化钠与CO2反应生成32g O2,则反应转移的电子数为2NA | |
| D. | 密闭容器中,标准状况下22.4L SO2和11.2LO2 在加热、有催化剂的条件下充分反应,容器中的分子总数为NA |
 实验室用FeSO4溶液和NaOH溶液反应制取Fe(OH)2,却很难看到稳定的白色沉淀.有同学设计了一种新的实验方法,能清楚看到生成白色沉淀及白色沉淀转化成红褐色沉淀的现象.请完成以下空白:
实验室用FeSO4溶液和NaOH溶液反应制取Fe(OH)2,却很难看到稳定的白色沉淀.有同学设计了一种新的实验方法,能清楚看到生成白色沉淀及白色沉淀转化成红褐色沉淀的现象.请完成以下空白: