��Ŀ����
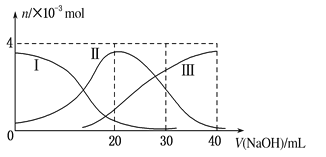
����Ŀ����ͼ(���Т����H2A�������HA���������A2��)����20mL0.2mol��L H2A��Һ�еμ�0.2mol��L NaOH��Һ������ͼʾ�жϣ�����˵����ȷ���ǣ� ��
A.H2A��ˮ�еĵ��뷽��ʽ�ǣ�H2A=H����HA����HA��![]() H����A2��
H����A2��
B.��V(NaOH)��20 mLʱ����Һ�и�����Ũ�ȵĴ�С˳��Ϊ��c(OH��) >c(H��)
C.��V(NaOH)��40 mLʱ����Һ��ˮ�ĵ���̶ȱȴ�ˮ��
D.��V(NaOH)��30 mLʱ����Һ�д������¹�ϵ��2c(H��)��c(HA��)��2c(H2A)��c(A2��)��2c(OH��)
���𰸡�C
��������
A�������H2A����V(NaOH)��0 mLʱ��H2A���Ӳ�Ϊ0��H2A��������ʣ�H2A��ˮ�еĵ��뷽��ʽ�ǣ�H2A![]() H����HA����HA��
H����HA����HA��![]() H����A2������A����
H����A2������A����
B����V��NaOH��=20 mLʱ��������ӦΪNaOH+H2A=NaHA+H2O����ҺΪNaHA��Һ������ͼ����Һ��A2�������ʵ�������H2A��HA���ĵ���̶ȴ���ˮ��̶ȣ���Һ�����ԣ�c(OH��) <c(H��)����B����
C����V(NaOH)��40 mLʱ����ҺΪNa2A��Һ��ǿ��������ˮ��ٽ�ˮ���룬��Һ��ˮ�ĵ���̶ȱȴ�ˮ��C��ȷ��
D����V��NaOH��=30mLʱ��������ӦΪNaOH+H2A=NaHA+H2O��NaHA+NaOH=Na2A+H2O����ҺΪ�����ʵ�����NaHA��Na2A�Ļ����Һ�����ݵ���غ�ã�c��Na����+c��H����=c��HA����+2c��A2����+c��OH�����٣������غ��֪��3c��HA����+3c��A2����+3c��H2A��=2c��Na�����ڣ�����2+�ڵã�2c��H����+c��HA����+3c��H2A���Tc��A2����+2c��OH��������D����
�ʴ�ѡC��

����Ŀ����ҵ�Ͽ���һ����̼�ϳɿ�������Դ�״���
(1)��֪����.3CO(g)��6H2(g) ![]() CH3CH��CH2(g)��3H2O(g) ��H1��-301.3kJ/mol��
CH3CH��CH2(g)��3H2O(g) ��H1��-301.3kJ/mol��
��.3CH3OH(g) ![]() CH3CH��CH2(g)��3H2O(g) ��H2��-31.0kJ/mol��
CH3CH��CH2(g)��3H2O(g) ��H2��-31.0kJ/mol��
��CO��H2�ϳ���̬�״����Ȼ�ѧ����ʽΪ___________________________________
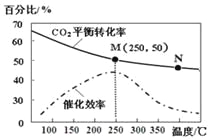
(2)ij����С����Cu2O/ZnO�������������£���500��ʱ���о���n(H2)��n(CO)�ֱ�Ϊ2��1��5��2ʱCO��ת���ʱ仯���(��ͼ1��ʾ)����ͼ�б�ʾn(H2)��n(CO)��2��1�ı仯����Ϊ________(��������a����������b��)��ԭ����_________________��

(3)ij����С�����ܱ������г���һ������CO��H2�ϳ���̬�״����ֱ���A��B���ֲ�ͬ���������·�����Ӧ��һ��ʱ�����CH3OH�IJ������¶ȵĹ�ϵ��ͼ2��ʾ������˵����ȷ����____________(��ѡ����ĸ)��
a.ʹ�ô���A�ܼӿ���ػ�ѧ��Ӧ���ʣ�������A��δ���뷴Ӧ
b.�ں��º�ѹ��ƽ����ϵ�г��������CH3OH�IJ��ʽ���
c.��2v(CO)����v(H2)��ʱ����Ӧ�ﵽƽ��״̬
(4)һ���¶��£����ݻ���Ϊ2L�����������ܱ������У������·�ʽ���뷴Ӧ�һ��ʱ���ﵽƽ�⡣
���� | �� | �� |
��Ӧ����ʼͶ���� | 2 mol CO��6 mol H2 | a mol CO��b mol H2��c mol CH3OH(g)(a��b��c����Ϊ��) |
��������ƽ��������ѹǿΪ��ʼʱ��![]() ������¶��£��÷�Ӧ��ƽ�ⳣ��K��_______��Ҫʹƽ��������������������ͬ��ֵ����������ȣ�����ʼʱά�ֻ�ѧ��Ӧ���淴Ӧ������У�����������c��ȡֵ��ΧΪ_____________________________________��
������¶��£��÷�Ӧ��ƽ�ⳣ��K��_______��Ҫʹƽ��������������������ͬ��ֵ����������ȣ�����ʼʱά�ֻ�ѧ��Ӧ���淴Ӧ������У�����������c��ȡֵ��ΧΪ_____________________________________��
(5)CO���ճ�����������ء�

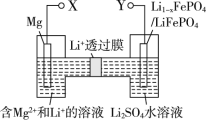
���������β����CO����������CO�����ǣ�����ԭ��������ȼ�ϵ�أ����е������������(Y2O3)�������(ZrO2)���壬�ܴ���O2�������ĵ缫��ӦʽΪ__________________��
��̼�������[(CH3O)2CO]����С����һ����ɫ������Ʒ����CO�ϳ�(CH3O)2CO����绯ѧ�ϳ�ԭ��Ϊ4CH3OH��2CO��O2![]() 2(CH3O)2CO��2H2O��װ����ͼ3��ʾ��д�������ĵ缫��Ӧʽ��________________________________________
2(CH3O)2CO��2H2O��װ����ͼ3��ʾ��д�������ĵ缫��Ӧʽ��________________________________________