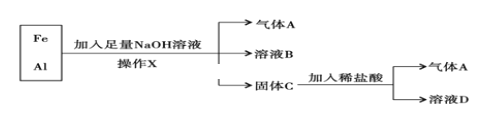
ћвƒњƒЏ»Ё
°Њћвƒњ°њ”…”Џ√ЊЇѕљрЊя”–”≤ґ»іу°Ґ√№ґ»–°°Ґ…Ґ»»–‘Ї√°Ґњє’р–‘Ї√µ»”≈“м–‘ƒ№Ћь±ї”√”Џ÷∆± Љ«±Њµзƒ‘Ќвњ«°ҐЊЇ»ь„‘––≥µ≥µЉ№µ»°£ѕ÷≥∆»°“їґ®÷ Ѕњµƒ√Њ¬ЅЇѕљр—щ∆ЈЈ≈»л500 mLѕ°ЅтЋб÷–£ђєћће»Ђ≤њ»№љв≤ҐЈ≈≥ц∆шће°£іэЈі”¶Ќк»ЂЇу£ђѕтЋщµ√»№“Ї÷–Љ”»лNaOH»№“Ї£ђ…ъ≥…≥Ѕµнµƒќп÷ µƒЅњ”лЉ”»лNaOH»№“ЇµƒћеїэєЎѕµ»зѕ¬ЌЉЋщ Њ°£
(1)Їѕљр÷–Alµƒ÷ Ѕњќ™__________________°£
(2)NaOH»№“Їµƒќп÷ µƒЅњ≈®ґ»ќ™__________________°£
(3)ѕ°ЅтЋбµƒќп÷ µƒЅњ≈®ґ»ќ™__________________°£
°Њір∞Є°њ5.4g 4.0 mol/L 0.8 mol/L
°Њљвќц°њ
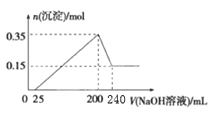
£®1£©ЄщЊЁЌЉѕуњ…÷™£ђ0°Ђ25 mLЈҐ…ъЋбЉо÷–ЇЌ£ђ25°Ђ200 mLЈҐ…ъјл„””лЉо…ъ≥…≥ЅµнµƒЈі”¶£ђ200°Ђ240mLЈҐ…ъAl(OH)3+NaOH=NaAlO2+2H2O£ђ200 mL ±…ъ≥…≥Ѕµн„оґа£ђ»№“Ї÷–µƒ»№÷ ќ™ЅтЋбƒ∆£ђ”…ЌЉѕуњ…÷™£ђ«в—хїѓ√Њµƒќп÷ µƒЅњќ™0.15 mol£ђ»№љвµƒ«в—хїѓ¬Ѕµƒќп÷ µƒЅњќ™£Ї0.35 mol-0.15 mol=0.2 mol£ђЄщЊЁ¬Ѕ‘≠„” ЎЇгњ…µ√Alµƒ÷ Ѕњ£ї
£®2£©ЄщЊЁЈі”¶Al(OH)3+NaOH=NaAlO2+2H2Oњ…«уµ√«в—хїѓƒ∆µƒќп÷ µƒЅњ≈®ґ»£ї
£®3£©»№“Ї„оЇуµƒ»№÷ ќ™ЅтЋбƒ∆£ђЄщЊЁ‘™ЋЎ ЎЇгњ…÷™£ђn(H2SO4) = n(SO42-) = n(Na+)/2 = n(NaOH)/2£ђЊЁіЋЈ÷ќц„чір°£
£®1£©√Њ¬ЅЇѕљр—щ∆ЈЈ≈»л500 mLѕ°ЅтЋб÷–£ђєћће»Ђ≤њ»№љв…ъ≥…√Њјл„”°Ґ¬Ѕјл„”£ђѕтіЋ»№“Ї÷–Љ”»лNaOH»№“Ї£ђі”…ъ≥…≥ЅµнЌЉѕуЈ÷ќцњ…÷™£ђ«в—хїѓƒ∆ћеїэі”0-25 mL ±√ї”–≥Ѕµн…ъ≥…£ђЋµ√ч‘≠»№“Ї÷–іж‘Џ«вјл„”ЉіЅтЋбєэЅњ£ђ200-250 mLґќ≥Ѕµн≤њЈ÷ѕы І£ђЈҐ…ъµƒЈі”¶ «£ЇAl(OH)3+OH-=AlO2-+2H2O£ђ”…¬Ѕ‘™ЋЎ ЎЇгµ√£Їn(Al) = n[Al(OH)3] = (0.35-0.15) mol = 0.2 mol£ђm(Al)= 0.2mol °Ѕ27g/mol = 5.4 g£ђ
є ір∞Є£Ї5.4 g£ї
£®2£©n(Al) = n[Al(OH)3] = n(OH-) = n(NaOH) = (250-200) mL°Ѕ10-3°Ѕc(NaOH) = 0.2mol£ђc(NaOH)= 4.0 mol/L£ї
£®3£©‘Џ200 mL ±£ђ≥Ѕµн ««в—хїѓ√ЊЇЌ«в—хїѓ¬Ѕ£ђ»№÷ ќ™ЅтЋбƒ∆£ђіЋ ±V(NaOH)=200 mL£ђn(NaOH)=0.2 L°Ѕ4.0 mol/L=0.8 mol£ђ‘тn(H2SO4) = n(SO42-) = n(Na+)/2= n(NaOH)/2 = 0.4 mol£ђc(H2SO4)= 0.4 mol/0.5 L=0.8 mol/L°£

 Їмєы„”»эЉґ≤в ‘ЊнѕµЅ–ір∞Є
Їмєы„”»эЉґ≤в ‘ЊнѕµЅ–ір∞Є њќћ√ЅЈЉ”≤вѕµЅ–ір∞Є
њќћ√ЅЈЉ”≤вѕµЅ–ір∞Є°Њћвƒњ°њ25°ж ±£ђ”–єЎќп÷ µƒµзјл∆љЇв≥£ э»зѕ¬£Ї
їѓ—І љ | CH3COOH | H2CO3 | H2SO3 |
µзјл∆љЇв≥£ э | K=1.8°Ѕ10£≠5 | K1=4.3°Ѕ10£≠7 K2=5.6°Ѕ10£≠11 | K1=1.5°Ѕ10£≠2 K2=1.02°Ѕ10£≠7 |
(1)«л–і≥цH2SO3µƒµзјл∆љЇв≥£ эK1µƒ±ніп љ£Ї________________°£
(2) ≥£ќ¬ѕ¬£ђљЂћеїэќ™10mL pH=2µƒі„Ћб»№“Ї”л—«ЅтЋб»№“ЇЈ÷±рЉ”’фЅуЋЃѕ° Ќ÷Ѕ1000mL£ђѕ° ЌЇу»№“ЇµƒpH£ђ«∞’я_____Їу’я£®ћо°∞£Њ°±°Ґ°∞£Љ°±їт°∞=°±£©°£
(3)“їґ®ћхЉюѕ¬£ђ±щі„ЋбЉ”ЋЃѕ° Ќєэ≥ћ÷–»№“ЇµЉµзƒ№Ѕ¶IЋжЉ”ЋЃћеїэV±дїѓ«ъѕя»з”“ЌЉЋщ Њ£ђ‘тa°Ґb°Ґc»эµг»№“Їі„Ћбµƒµзјл≥ћґ»”…іуµљ–°ќ™____________________°£

(4)ѕ¬Ѕ–јл„”CH3COO£≠°ҐCO32£≠°ҐHSO3£≠°ҐSO32£≠‘Џ»№“Ї÷–љбЇѕH£Ђµƒƒ№Ѕ¶”…іуµљ–°µƒєЎѕµќ™___________°£
(5)ћеїэѕаЌђ°Ґc(H£Ђ)ѕаЌђµƒҐўCH3COOH£їҐЏHCl£їҐџH2SO4 »э÷÷Ћб»№“ЇЈ÷±р”лЌђ≈®ґ»µƒNaOH»№“ЇЌк»Ђ÷–ЇЌ ±£ђѕыЇƒNaOH»№“Їµƒћеїэ”…іуµљ–°µƒ≈≈Ѕ–Ћ≥–т «_____(ћо–тЇ≈)°£
(6)“—÷™£ђH£Ђ(aq) + OH£≠(aq) == H2O(l) ¶§H =£≠57.3 kJ/mol°£ µ—й≤вµ√ѕ°і„Ћб”лѕ°NaOH»№“ЇЈі”¶…ъ≥…1 mol H2O ±Ј≈≥ц57 kJµƒ»»£ђ‘ті„Ћб»№“Ї÷–£ђі„Ћбµзјлµƒ»»їѓ—ІЈљ≥ћ љќ™________________°£
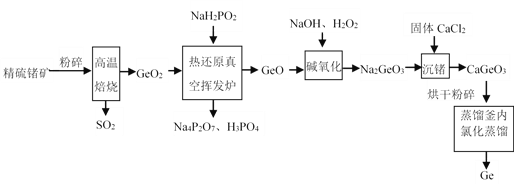
°Њћвƒњ°њ’а «“ї÷÷÷Ў“™µƒ∞лµЉће≤ƒЅѕ°£є§“µ…ѕ”√ЊЂЅт’ању£®÷ч“™≥…Ј÷ќ™GeS2£ђ‘”÷ ≤їЈі”¶£©÷∆»°Ge£ђ∆дє§“’Ѕч≥ћ»зЌЉЋщ Њ£Ї
їЎірѕ¬Ѕ–ќ ћв£Ї
£®1£©њ™ ЉљЂЊЂЅт’ањуЈџЋйµƒƒњµƒ «______°£
£®2£©Єяќ¬±Ї…’ЊЂЅт’ањуµƒїѓ—ІЈљ≥ћ љќ™______°£
£®3£©»»їє‘≠’жњ’ї”ЈҐ¬ѓƒЏЈі”¶µƒїѓ—ІЈљ≥ћ љќ™______°£
£®4£©‘Џ≥Ѕ’аєэ≥ћ÷–£ђµ±ќ¬ґ»ќ™90°ж£ђpHќ™14 ±£ђЉ”ЅѕЅњ£®CaCl2/Ge÷ Ѕњ±»£©ґ‘≥Ѕ’аµƒ”∞ѕм»з±нЋщ Њ£ђ—°‘с„оЉ—Љ”ЅѕЅњќ™______£®ћо°∞10-15°±°∞15-20°±їт°∞20-25°±£©£ђјн”… «______°£
±аЇ≈ | Љ”ЅѕЅњ£®CaCl2/Ge£© | ƒЄ“Їћеїэ £®mL£© | єэ¬ЋЇу“ЇЇђ’а£®mg/L£© | єэ¬ЋЇу“Ї pH | ’а≥Ѕµн¬ £®%£© |
1 | 10 | 500 | 76 | 8 | 93.67 |
2 | 15 | 500 | 20 | 8 | 98.15 |
3 | 20 | 500 | 2 | 11 | 99.78 |
4 | 25 | 500 | 1.5 | 12 | 99.85 |
£®5£©ƒ≥ќ¬ґ» ±£ђ≥Ѕ’аµ√µљµƒCaGeO3‘ЏЋЃ÷–µƒ≥Ѕµн»№љв∆љЇв«ъѕя»зЌЉЋщ Њ°£ѕ¬Ѕ–ЋµЈ®інќуµƒ «______°£
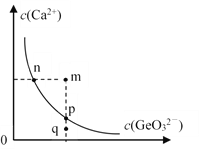
a£Ѓnµг”лpµгґ‘”¶µƒKspѕаµ»
b£ЃЌ®єэ’фЈҐњ…“‘ є»№“Ї”…qµг±дµљpµг
c£ЃqµгќёCaGeO3≥Ѕµн…ъ≥…
d£ЃЉ”»лNa2GeO3њ…“‘ є»№“Ї”…nµг±дµљmµг
£®6£©CaGeO3”л«њЋбЈі”¶њ…µ√µљH2GeO3°£0.l molL£≠1µƒNaHGeO3»№“ЇpH_____£®ћо°∞£Њ°±°∞=°±їт°∞£Љ°±£©7£ђ≈–ґѕјн”… «______£®Ќ®єэЉ∆Ћг±»љѕ£©°££®25°ж ±£ђH2GeO3µƒKa1=1.7°Ѕ10£≠9£ђKa2=1.9°Ѕ10£≠13°££©