��Ŀ����
�����£���1 mol N2��3 mol H2�����Ϊ2 L�������л�ϣ��������·�Ӧ��N2(g)��3H2(g)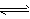 2NH3(g),2 sʱ���NH3���������Ϊ25%��������˵���в���ȷ����
2NH3(g),2 sʱ���NH3���������Ϊ25%��������˵���в���ȷ����
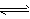 2NH3(g),2 sʱ���NH3���������Ϊ25%��������˵���в���ȷ����
2NH3(g),2 sʱ���NH3���������Ϊ25%��������˵���в���ȷ����[ ]
A����N2Ũ�ȵļ��ٱ�ʾ��ƽ����Ӧ����Ϊ0.2 mol��L��1��s��1
B��2 sʱN2��ת����Ϊ40%
C��2 sʱ���������n(N2)��n(H2)��n(NH3)��3��9��4
D��2 s ʱNH3��Ũ��Ϊ0.4 mol��L��1
B��2 sʱN2��ת����Ϊ40%
C��2 sʱ���������n(N2)��n(H2)��n(NH3)��3��9��4
D��2 s ʱNH3��Ũ��Ϊ0.4 mol��L��1
A

��ϰ��ϵ�д�
 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
�����Ŀ