��Ŀ����
13��������A��һ��������������D��3������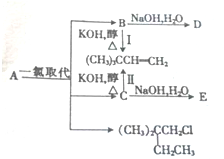 ��1��A�ķ���ʽΪC6H14��1molA��ȫȼ������O29.5mol��
��1��A�ķ���ʽΪC6H14��1molA��ȫȼ������O29.5mol����2��D�Ľṹ��ʽΪ��CH3��3CCH2CH2OH����Ӧ��ķ�Ӧ����Ϊ��ȥ��Ӧ��
��3����Ӧ��Ļ�ѧ����ʽΪ��CH3��3CCHClCH3+KOH$��_{��}^{��}$��CH3��3CCH=CH2+KCl+H2O
��4��E��ͬ���칹���У��ܷ���������Ӧ����17�֣�������E�����������к���4������ͬ���칹��Ľṹ��ʽΪ��CH3��2CHCOH��CH3��2��
���� �������и�����ת����ϵ��A��һ��������һ��ȡ��������B��C��B��CΪһ�ȴ��B��C���������صĴ���Һ�з�����ȥ��Ӧ�ã�CH3��3CCH=CH2����AΪ��CH3��3CCH2CH3��������D��3������B��������������Һ�з���ˮ���D����DΪ��CH3��3CCH2CH2OH������BΪ��CH3��3CCH2CH2Cl����CΪ��CH3��3CCHClCH3������EΪ��CH3��3CCHOHCH3���ݴ˴��⣮
��� �⣺�������и�����ת����ϵ��A��һ��������һ��ȡ��������B��C��B��CΪһ�ȴ��B��C���������صĴ���Һ�з�����ȥ��Ӧ�ã�CH3��3CCH=CH2����AΪ��CH3��3CCH2CH3��������D��3������B��������������Һ�з���ˮ���D����DΪ��CH3��3CCH2CH2OH������BΪ��CH3��3CCH2CH2Cl����CΪ��CH3��3CCHClCH3������EΪ��CH3��3CCHOHCH3��
��1��AΪ��CH3��3CCH2CH3��A�ķ���ʽΪC6H14��1molA��ȫȼ������O2�����ʵ���Ϊ$\frac{6��2+14��\frac{1}{2}}{2}$mol=9.5mol���ʴ�Ϊ��C6H14��9.5��
��2����������ķ�����֪��DΪ��CH3��3CCH2CH2OH����Ӧ��ķ�Ӧ����Ϊ��ȥ��Ӧ���ʴ�Ϊ����CH3��3CCH2CH2OH����ȥ��Ӧ��
��3����Ӧ��Ļ�ѧ����ʽΪ��CH3��3CCHClCH3+KOH$��_{��}^{��}$��CH3��3CCH=CH2+KCl+H2O���ʴ�Ϊ����CH3��3CCHClCH3+KOH$��_{��}^{��}$��CH3��3CCH=CH2+KCl+H2O��
��4��EΪ��CH3��3CCHOHCH3��E��ͬ���칹���У��ܷ���������Ӧ��˵�����ǻ������������Ľṹ��CH3CH2CH2CH2CH2CH2OH��CH3CH2CH2CH2CHOHCH3��CH3CH2CH2CHOHCH2CH3��CH2OHCH2CH2CH��CH3��2��CH2OHCH2CH2CH��CH3��2��CH3CHOHCH2CH��CH3��2��CH3CH2CHOHCH��CH3��2��CH3CH2CH2COH��CH3��2��CH3CH2CH2CH��CH3��CH2OH��CH3CH2CH2CH��CH3��CH2CH2OH��CH3CH2CH2CH��CH3��CHOHCH3��CH3CH2CH2COH��CH3��CH2CH3��CH3CH2CH2CH��CH2OH��CH2CH3����CH3��2CHCOH��CH3��2����CH3��2CHCH��CH2OH��CH3����CH3��3CCH2CH2OH��CH2OHC��CH3��2CH2CH3������17 �֣����к���4������ͬ���칹��Ľṹ��ʽΪ��CH3��2CHCOH��CH3��2��
�ʴ�Ϊ��17����CH3��2CHCOH��CH3��2��
���� ���⿼���л�����ƶ����л�������ʣ���ȷת���еķ�Ӧ���������ʵĽṹ�仯�ǽ����Ĺؼ�����Ŀ�Ѷ��еȣ�

 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д� Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д� ����ͼ��ʾװ�ý�������ʵ�飬ʵ������Ԥ������һ�µ��ǣ�������
����ͼ��ʾװ�ý�������ʵ�飬ʵ������Ԥ������һ�µ��ǣ�������| ѡ�� | �������� | �������� | Ԥ��������� |
| A | ��̪��Һ | Ũ��ˮ | ��ɫ���ɫ |
| B | ʪ��첼�� | ������ˮ | �첼����ɫ |
| C | ����KI��Һ | Ũ���� | �����Ա仯 |
| D | ��������Һ | Ũ���� | �а�ɫ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | Y��Z��X | B�� | Z��X��Y | C�� | X��Y��Z | D�� | Y��X��Z |
| A�� | ��0.5mol��ԭ�� | B�� | ��1�������� | ||
| C�� | ��������14g | D�� | Լ��6.02��1023�������� |
 Ԫ�����ڱ�����ָ������Ѱ�Һͺϳɳ��µ����ʣ�������ͷǽ����ֽ��߸�����Ԫ�ؿ���������뵼����ϣ��ǿ�ѧ�о����ȵ�
Ԫ�����ڱ�����ָ������Ѱ�Һͺϳɳ��µ����ʣ�������ͷǽ����ֽ��߸�����Ԫ�ؿ���������뵼����ϣ��ǿ�ѧ�о����ȵ� ��
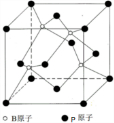
�� ��������в�״�ṹ��ÿ��ṹ��ͼ��ʾ��ÿĦ�������������������ĿΪ3NA����NAΪ����٤��������ֵ��
��������в�״�ṹ��ÿ��ṹ��ͼ��ʾ��ÿĦ�������������������ĿΪ3NA����NAΪ����٤��������ֵ�� X��Y��Z��RΪ������Ԫ�أ�ԭ��������������X��һ�ֵ�������Ȼ������Ӳ�����ʣ�Y�ĵ����ڿ����к�����ߣ�Z ���������dz��������������R ��̬ԭ�������ɶԵ��ӵ���Ŀ��δ�ɶԵ��� ����Ŀ��ȣ�
X��Y��Z��RΪ������Ԫ�أ�ԭ��������������X��һ�ֵ�������Ȼ������Ӳ�����ʣ�Y�ĵ����ڿ����к�����ߣ�Z ���������dz��������������R ��̬ԭ�������ɶԵ��ӵ���Ŀ��δ�ɶԵ��� ����Ŀ��ȣ� 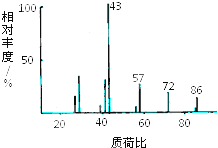 ij�л��ﺬ��C��H��O����Ԫ�أ���������ͼ��ʾ����4.3g���л�����O2�г��ȼ�գ�ʹ����������ͨ������Ũ����ͼ�ʯ�ң�Ũ��������2.7g����ʯ������8.8g����
ij�л��ﺬ��C��H��O����Ԫ�أ���������ͼ��ʾ����4.3g���л�����O2�г��ȼ�գ�ʹ����������ͨ������Ũ����ͼ�ʯ�ң�Ũ��������2.7g����ʯ������8.8g����