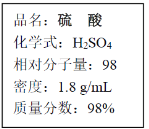
��Ŀ����
����Ŀ������˵��������ʵ���ǣ� ��
A. ��������ˮ���������c(H+)=10-12mol/L����Һ�м������۶��ܹ���������
B. ��0.2000 /L NaOH����Һ�ζ�HCl��CH3COOH�Ļ����Һ�����Һ���������Ũ�Ⱦ�ԼΪ0.1mol/L����������ʱ����Һ�е���δ����ȫ�к�
C. �������ͭ��Һһ��ʱ���������ͭ���岻����ʹ��Һ�ָ���ԭ����Ũ��
D. ��NaAlO2��Na2CO3�Ļ����Һ����εμ�����ֱ����������ʼû�г�������������������������ʧ
���𰸡�B
��������
A����������ˮ���������c(H��)=10-12mol��L-1������������ˮ�ĵ��룬����Ϊ�ᣬҲ����Ϊ����������Ϊ���ᣬ������Al��Ӧ������H2����A���������⣻
B��HClΪǿ�ᣬCH3COOHΪ���ᣬ�ζ�ʱ��������ΪHCl����NaOH��Ӧ����HCl�ζ�����ٵζ�CH3COOH�����CH3COOH��NaOH�ζ�������ʱ����Һ�е�����ΪNaCl��CH3COOH��CH3COONa��CH3COOHδ����ȫ�кͣ���B��ȷ��
C�����CuSO4��Һ������ܷ�ӦΪ2CuSO4+2H2O ![]() 2Cu+O2��+2H2SO4������CuO��ʹ��Һ�ָ���ԭ����Ũ�ȣ���C����
2Cu+O2��+2H2SO4������CuO��ʹ��Һ�ָ���ԭ����Ũ�ȣ���C����
D��AlO2-���H+����ǿ��CO32-�������Һ����ε������ᣬAlO2-����H+����AlO2-+H++H2O=Al(OH)3��������ʼʱ�������������������ɣ���D����
��ѡB��

����Ŀ����֪25��ʱ�й�����ĵ���ƽ�ⳣ�����������й�˵����ȷ���ǣ� ��
���ữѧʽ | CH3COOH | HCN | H2CO3 |
����ƽ�ⳣ�� | 1.8��10-5 | 4.9��10-10 | K1��4.3��10-7 K2��5.6��10-11 |
A. �����ʵ���Ũ�ȵĸ���ҺpH��ϵΪ��pH(NaCN)��pH(Na2CO3)��pH(CH3COONa)
B. ����������μ�ˮ������Һ�ĵ����ԡ�����ĵ���̶ȡ�pH����������С
C. NaCN��ͨ������CO2�����Ļ�ѧ��ӦΪ��NaCN+CO2+H2O=HCN+NaHCO3
D. ϡ��HCN��Һ�����У�![]() ��С
��С
����Ŀ���±�ΪԪ�����ڱ���һ���֡�
̼ | �� | Y | |
X | �� | Z |
�ش��������⣺
��1��ZԪ�������ڱ��е�λ��Ϊ___��
��2��������ʵ��˵��YԪ�صķǽ����Ա�SԪ�صķǽ�����ǿ����___��
a��Y������H2S��Һ��Ӧ����Һ�����
b����������ԭ��Ӧ�У�1molY���ʱ�1molS�õ��Ӷ�
c��Y��S��Ԫ�صļ��⻯�����ȷֽ⣬ǰ�ߵķֽ��¶ȸ�
��3��X��Z��Ԫ�صĵ��ʷ�Ӧ����1molX����ۻ�����ָ������£�����687kJ����֪�û�������ۡ��е�ֱ�Ϊ-69���58�棬д���÷�Ӧ���Ȼ�ѧ����ʽ___��
��4��̼��þ�γɵ�1mol������Q��ˮ��Ӧ������2molMg(OH)2��1mol��������������̼��������Ϊ9��1�����ĵ���ʽΪ___��Q��ˮ��Ӧ�Ļ�ѧ����ʽΪ__��
����Ŀ��800��ʱ����2L���ܱ������з�����Ӧ��2NO(g)+O2(g)![]() 2NO2��n(NO)��ʱ��ı仯���±���ʾ��
2NO2��n(NO)��ʱ��ı仯���±���ʾ��
ʱ���Ms | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)�Mmol | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
��1����0��3s�ڣ���NO��ʾ��ƽ����Ӧ������(NO)��_____________��
��2��ͼ�б�ʾNOŨ�ȱ仯��������_______(����ĸ����)��
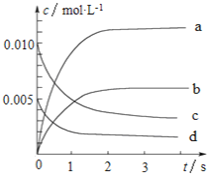
��3����ƽ��ʱNO��ת����Ϊ________��
��4����˵���÷�Ӧ�Ѵﵽƽ��״̬����_________(�����)��
A������������ɫ���ֲ���
B�����������ܶȱ��ֲ���
C������ (NO2)��2���� (O2)
D����������ƽ����Է����������ֲ���
��5�������µ�850�棬��ƽ���n(NO)��n(NO2)����Ӧ��________(��������Ӧ�����������淴Ӧ������)�ƶ���
��6������һ��������0.2molNO������������Ӧ����ƽ��ʱ��÷ų�������ΪakJ����ʱNOת����Ϊ80%����2molNO������ȫ��Ӧ�ų�������Ϊ____________��