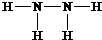
��Ŀ����
X��Y��Z��L��M����Ԫ�ص�ԭ��������������X��Y��Z��L����ɵ����ʵĻ���Ԫ�أ�M����������õĽ���������L�����γ����ֻ����
��ش��������⣺
��1��M��Ԫ�����ڱ��е�λ��Ϊ______
��2��X �ֱ���Z��L �γɵĻ�����A��B������18e-��A��һ��6ԭ�ӷ��ӣ����������������ȼ�ϣ�B��һ�ֳ���ǿ����������֪16gҺ̬A��Һ̬B��ַ�Ӧ����һ��Һ̬10���ӷ���C��һ����̬���ʣ����ų�838kJ������д��A��B��Ӧ���Ȼ�ѧ����ʽ______
��3����֪��YZ��2�������е�ԭ�Ӷ�����8���ӣ���д����ṹʽ______����YZ��2������X2��������XYZ����ˮ��Һ��һ���ᣬijŨ�ȸ���ļ��Σ�KYZ����Һ��ʹ��̪��Һ�Ժ�ɫ���������ӷ���ʽ��ʾԭ��______
��4��YL2�ĵ���ʽ��______������ӵĿռ乹����______
��5��M�ڸ����¿�����C��Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ______
ijͬѧ����Ӧ�õ��ĺ�ɫ��ĩȫ������������������Һ�У����������������ݲ�������Һ���ػ�ɫ����ͬѧ�ж���Һ��һ��ͬʱ��������M�Ľ��������ӣ�����ͬѧ�������ػ�ɫ��Һ1mL���뵽2mL A��Ũ��Һ�У�Ԥ�ƻῴ����ʵ��������______��
�⣺X��Y��Z��L��M����Ԫ�ص�ԭ��������������X��Y��Z��L����ɵ����ʵĻ���Ԫ�أ���ɵ����ʵĻ���Ԫ����C��H��O��N����X��Y��Z��L��ԭ������������������X��HԪ�أ�Y��CԪ�أ�Z��NԪ�أ�L��OԪ�أ�M����������õĽ���������L�����γ����ֻ������M��Fe��
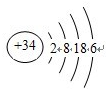
��1��Mԭ�Ӻ�����26�����ӣ�������4�����Ӳ㣬�������2�����ӣ�λ�ڵ������ڵ�VIII�壬
�ʴ�Ϊ���������ڵ�VIII�壻
��2��X�ֱ���Z��L �γɵĻ�����A��B������18e-��A��һ��6ԭ�ӷ��ӣ����������������ȼ�ϣ�����A��N2H4��B��һ�ֳ���ǿ��������B��H2O2����֪16gҺ̬A��Һ̬B��ַ�Ӧ����һ��Һ̬10���ӷ���H2O��һ����̬���ʵ��������ų�838kJ������16gN2H4�����ʵ���=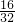 mol=0.5mol��0.5molN2H4��ȫ��Ӧ�ų�838kJ��������1molA��ȫ��Ӧ�ų�1676kJȼ�ϣ��������Ȼ�ѧ��Ӧ����ʽΪ��N2H4��l��+2H2O2��l��=4H2O��l��+N2��g����H=-1676kJ/mol��
mol=0.5mol��0.5molN2H4��ȫ��Ӧ�ų�838kJ��������1molA��ȫ��Ӧ�ų�1676kJȼ�ϣ��������Ȼ�ѧ��Ӧ����ʽΪ��N2H4��l��+2H2O2��l��=4H2O��l��+N2��g����H=-1676kJ/mol��
�ʴ�Ϊ��N2H4��l��+2H2O2��l��=4H2O��l��+N2��g����H=-1676kJ/mol��
��3����CN��2�������е�ԭ�Ӷ�����8���ӣ���̼ԭ���γ��ĸ����ۼ�����ԭ���γ��������ۼ���������ṹ��ʽΪ��N��C-C��N����CN��2������H2��������HCN����ˮ��Һ��һ���ᣬijŨ�ȸ���ļ��Σ�KCN����Һ��ʹ��̪��Һ�Ժ�ɫ���軯����ǿ�������Σ�������ˮ���ʹ����Һ�ʼ��ԣ�ˮ�ⷽ��ʽΪ��CN-+H2O=HCN+OH-��
�ʴ�Ϊ��N��C-C��N��CN-+H2O=HCN+OH-��
��4��CO2�ĵ���ʽΪ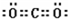 ��������̼��ֱ���ͷ��ӣ��ʴ�Ϊ��
��������̼��ֱ���ͷ��ӣ��ʴ�Ϊ�� ��ֱ���ͣ�
��ֱ���ͣ�
��5�������£�����ˮ������Ӧ������������������������Ӧ����ʽΪ��3Fe+4H2O��g�� Fe3O4+4H2��A��Ũ��Һ���л�ԭ�ԣ��ܱ��������������ɵ��������Կ������������д������ݳ��֣��ʴ�Ϊ��3Fe+4H2O��g��
Fe3O4+4H2��A��Ũ��Һ���л�ԭ�ԣ��ܱ��������������ɵ��������Կ������������д������ݳ��֣��ʴ�Ϊ��3Fe+4H2O��g�� Fe3O4+4H2���д������ݳ��֣�
Fe3O4+4H2���д������ݳ��֣�
������X��Y��Z��L��M����Ԫ�ص�ԭ��������������X��Y��Z��L����ɵ����ʵĻ���Ԫ�أ���ɵ����ʵĻ���Ԫ����C��H��O��N����X��Y��Z��L��ԭ������������������X��HԪ�أ�Y��CԪ�أ�Z��NԪ�أ�L��OԪ�أ�M����������õĽ���������L�����γ����ֻ������M��Fe������Ԫ�ػ�����֪ʶ�������
���������⿼��Ԫ�ػ���������ʣ���ȷ�ƶ�Ԫ���ǽⱾ��ؼ����漰��֪ʶ��϶࣬�ѶȲ���ע�����֪ʶ�Ļ��ۺ����ã�
��1��Mԭ�Ӻ�����26�����ӣ�������4�����Ӳ㣬�������2�����ӣ�λ�ڵ������ڵ�VIII�壬
�ʴ�Ϊ���������ڵ�VIII�壻
��2��X�ֱ���Z��L �γɵĻ�����A��B������18e-��A��һ��6ԭ�ӷ��ӣ����������������ȼ�ϣ�����A��N2H4��B��һ�ֳ���ǿ��������B��H2O2����֪16gҺ̬A��Һ̬B��ַ�Ӧ����һ��Һ̬10���ӷ���H2O��һ����̬���ʵ��������ų�838kJ������16gN2H4�����ʵ���=
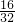 mol=0.5mol��0.5molN2H4��ȫ��Ӧ�ų�838kJ��������1molA��ȫ��Ӧ�ų�1676kJȼ�ϣ��������Ȼ�ѧ��Ӧ����ʽΪ��N2H4��l��+2H2O2��l��=4H2O��l��+N2��g����H=-1676kJ/mol��
mol=0.5mol��0.5molN2H4��ȫ��Ӧ�ų�838kJ��������1molA��ȫ��Ӧ�ų�1676kJȼ�ϣ��������Ȼ�ѧ��Ӧ����ʽΪ��N2H4��l��+2H2O2��l��=4H2O��l��+N2��g����H=-1676kJ/mol���ʴ�Ϊ��N2H4��l��+2H2O2��l��=4H2O��l��+N2��g����H=-1676kJ/mol��
��3����CN��2�������е�ԭ�Ӷ�����8���ӣ���̼ԭ���γ��ĸ����ۼ�����ԭ���γ��������ۼ���������ṹ��ʽΪ��N��C-C��N����CN��2������H2��������HCN����ˮ��Һ��һ���ᣬijŨ�ȸ���ļ��Σ�KCN����Һ��ʹ��̪��Һ�Ժ�ɫ���軯����ǿ�������Σ�������ˮ���ʹ����Һ�ʼ��ԣ�ˮ�ⷽ��ʽΪ��CN-+H2O=HCN+OH-��
�ʴ�Ϊ��N��C-C��N��CN-+H2O=HCN+OH-��
��4��CO2�ĵ���ʽΪ
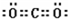 ��������̼��ֱ���ͷ��ӣ��ʴ�Ϊ��
��������̼��ֱ���ͷ��ӣ��ʴ�Ϊ�� ��ֱ���ͣ�
��ֱ���ͣ���5�������£�����ˮ������Ӧ������������������������Ӧ����ʽΪ��3Fe+4H2O��g��
 Fe3O4+4H2��A��Ũ��Һ���л�ԭ�ԣ��ܱ��������������ɵ��������Կ������������д������ݳ��֣��ʴ�Ϊ��3Fe+4H2O��g��
Fe3O4+4H2��A��Ũ��Һ���л�ԭ�ԣ��ܱ��������������ɵ��������Կ������������д������ݳ��֣��ʴ�Ϊ��3Fe+4H2O��g�� Fe3O4+4H2���д������ݳ��֣�
Fe3O4+4H2���д������ݳ��֣�������X��Y��Z��L��M����Ԫ�ص�ԭ��������������X��Y��Z��L����ɵ����ʵĻ���Ԫ�أ���ɵ����ʵĻ���Ԫ����C��H��O��N����X��Y��Z��L��ԭ������������������X��HԪ�أ�Y��CԪ�أ�Z��NԪ�أ�L��OԪ�أ�M����������õĽ���������L�����γ����ֻ������M��Fe������Ԫ�ػ�����֪ʶ�������
���������⿼��Ԫ�ػ���������ʣ���ȷ�ƶ�Ԫ���ǽⱾ��ؼ����漰��֪ʶ��϶࣬�ѶȲ���ע�����֪ʶ�Ļ��ۺ����ã�

��ϰ��ϵ�д�
 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�
�����Ŀ