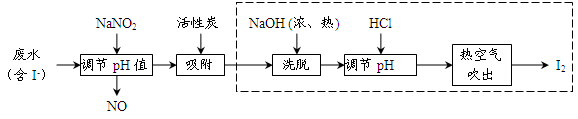
��Ŀ����
��9�֣����������( Na2S2O3)�����������Լ������ﻹԭ���������ȡ������ֽ⡣��ҵ�Ͽ��÷�Ӧ��2Na2S+Na2CO3+4SO2=3Na2S2O3 +CO2�Ƶá�ʵ����ģ��ù�ҵ���̵�װ����ͼ��ʾ��
�ش��������⣺
��1��b�з�Ӧ�����ӷ���ʽΪ__________________��c���Լ�Ϊ_____________________��
��2����Ӧ��ʼ��c�����л��Dz��������ֱ���塣�˻�������____________________��
��3��d�е��Լ�Ϊ____________��
��4��ʵ����Ҫ����SO2�������ʣ����Բ�ȡ�Ĵ�ʩ��______________________��д����������
��5��Ϊ�˱�֤��������ƵIJ�����ʵ����ͨ���SO2,���ܹ�����ԭ����_______________________��
(1)SO32-+2H+= H2O +SO2��;��HSO3��+H+=SO2��+H2O�����ƺ�̼���ƵĻ����Һ
��2����3��NaOH��Һ ��4�����Ʒ�Ӧ�¶ȡ�������ĵμ��ٶȣ���������Ũ�ȵȣ�
��5����SO2��������Һ�����ԣ�����ֽ�
�������������(1)װ��b����ȡSO2��װ�á���b�з�Ӧ�����ӷ���ʽΪSO32-+2H+= H2O +SO2��;��HSO3��+H+=SO2��+H2O�����ݷ�Ӧԭ����֪��c�е��Լ������ƺ�̼���ƵĻ����Һ��(2)��Ӧ��ʼ��c���ȷ�����Ӧ��H2O+SO2+Na2S=H2S+ Na2SO3; SO2+2H2S="3S��+" 2H2O.S�Dz�����ˮ�ĵ���ɫ���ʡ�����л��Dz��������ֱ���壬������Ӧ��2Na2S+Na2CO3+4SO2=3Na2S2O3 +CO2��(3) H2S ��SO2���Ǵ�����Ⱦ��������Ƕ����������壬�ܹ�������Ӧ��������d�е��Լ�Ϊǿ��NaOH��Һ��Ϊ��ֹ��������ķ������ڵ����ܵ�ĩ�˰�װ��һ������ܡ�(4)Ӱ�컯ѧ��Ӧ���ʵ����ص�������Ũ�ȡ��¶ȡ��μ��ٶȵȡ���ʵ����Ҫ����SO2�������ʣ����Բ�ȡ�Ĵ�ʩ�п��Ʒ�Ӧ�¶ȡ�������ĵμ��ٶȻ�������Ũ�ȵȡ���5�����������( Na2S2O3)��ǿ�������Σ�������������Ӧ��������SO2��������Һ�����ԣ��������ֽ⡣��˲��ܹ�����
���㣺�������ʵ��Ʊ�ԭ������Ӧ�����Ŀ��ơ��Լ���ѡ�����������������ʽ����д��֪ʶ��

����ʵ���ܴﵽԤ��Ŀ�ĵ���
| ��� | ʵ������ | ʵ��Ŀ�� |
| A | ��CO2����HC1���ʣ�ͨ�뱥��NaHCO3��Һ�� | ��ȥHC1 |
| B | ������������Ӧ����Թ��м���ϡ��ˮ | ��ȥ�Թ��ڲ����� |
| C | ���������ˮ������Һ��ֱ�Ӽ������Ƶ�Cu(OH)2�������� | ��������Ƿ�ˮ�� |
| D | ������FeC12������ˮ�ܽ⣬��ϡ�����ữ���ٵμ�KSCN��Һ | ����FeCl2�Ƿ���� |
��14�֣�����34Se�����ڣ�52Te�����ǵ�VIA��Ԫ�أ����Ƿֲ��ڵؿ��е�ϡ��Ԫ�ء���ҵ�����ķ��ϣ���Ҫ�ɷ������ڡ�̼��ͭ�����Ͻ𣩻��վ��������������£�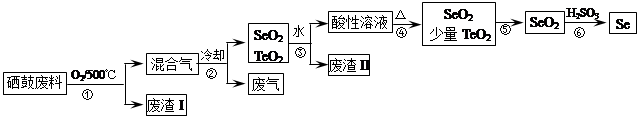
��֪��
| �������� | �۵� | �е� | ���� | �ܽ��� |
| SeO2 | 340�� | 684�� | 315�� | ������ˮ |
| TeO2 | 733�� | 1260�� | 450�� | ����ˮ |
��2���������ͨ�������ʹ���ķ��Ϸ��ڣ�Ŀ����______��
��3����������Ҫ�ɷ���______������II����Ҫ�ɷ���______��
��4�����������Ҫ��Ӧ�Ļ�ѧ����ʽ��______�������Ӧ�Ļ�ѧ����ʽ��______��
��5�����ݱ������ݣ�����������˵ķ��뷽����______��
�����������һ�ֿɴٽ�����������Ӫ�����ʡ���������ƿ�ͨ�����·�Ӧ�Ƶã�
C6H12O6(������)��Br2��H2O��C6H12O7(��������)��2HBr
2C6H12O7(��������)��CaCO3��Ca(C6H11O7)2(���������)��H2O��CO2
������ʵ��ܽ��Լ��±���
| �������� | ��������� | �������� | �廯�� | �Ȼ��� |
| ˮ�е��ܽ��� | ��������ˮ ��������ˮ | ���� | ���� | ���� |
| �Ҵ��е��ܽ��� | �� | �� | ���� | ���� |

��ش��������⣺
��1���ڢٲ�����ˮ����������ʱ������װ������ʵ���________��
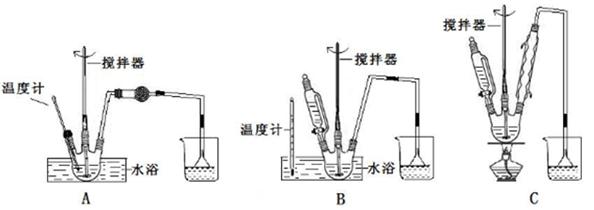
�Ʊ���������ƵĹ����У������ǵ�����Ҳ���������Լ����������������ʺϵ���________��
A������Cu(OH)2����Һ B������KMnO4��Һ
C��O2������������ø D��[Ag(NH3)2]OH��Һ
��2���ڢڲ���ַ�Ӧ��CaCO3��������ʣ�࣬��Ŀ����________����ʵ���в�����CaCl2���CaCO3��������________��
��3���ڢ۲�����ȹ��ˣ���ԭ����________��
��4���ڢܲ������Ҵ���������________��
��5���ڢ��У�����ϴ�Ӽ�����ʵ���________��
A����ˮ B����ˮ C���Ҵ� D���Ҵ���ˮ�����Һ
��16�֣�ij��ѧ��ȤС���ڽ������ڿ�����ȼ�յ�ʵ�飬��д������������ȼ�յĻ�ѧ����ʽ�� ��ʵ���ͬѧ�Ƿ��ֳ��˵õ���ɫ�����⣬�����л��к�ɫ���ʡ���ȤС���ͬѧ�Բ����ĺ�ɫ�������ʽ���ʵ��̽����
�������ϣ�
��ҵ�ϲ����������������õ������ơ�ʵ�����н����Ƶı�ǩ��Ҫ�������£� 
������룺
����1���Ƹ���ú�ͣ�ú�͵IJ���ȫȼ�տ�������̼���ʣ�����2�� ���Ʒ�ӦҲ��������̼���ʣ�
����3�� �� ����4������
���ʵ�鷽������֤���룺
| ʵ��Ŀ�� | ʵ�鲽�� | ʵ������ | ���� |
| ��֤����1 | ��һС���ƴ�ú����ֱ��ȡ������ȼ�� ȡ��һС����ȥ��Ƥ�����ɾ�ú�͵��Ƽ���ȼ�� | | ��ɫ�����к� ��̼���� |
| ��֤����3 | | | ��ɫ������ ������Ԫ�� |
ͬѧ���˽��������ʯ������ȼ�գ���ʯ��������Ҳ�������ɺ����ĺ�ɫ���ʣ�������������ʯ������ȼ��̽�����ڿ�����ȼ�ղ����Ƿ������˵��ԭ�� ��
�����ʵ����֤����2����Ҫ��д����������̣��� ��
��20�֣�ij�о���ѧϰС����ʵ����������0.20mol��L-1���������Һ��Ȼ������ζ�ijδ֪Ũ�ȵ�����������Һ��
�����ƴ���Һ����1��7 g������������(�������ᷴӦ)�Ĺ����ռ���Ʒ���Ƴ�200 mL��Һ���������Ҫ�����������ձ���200 mL����ƿ����Ͳ������ �p ��
�Ƶζ���
��ʢװ0.20mol��L-1�����ҺӦ���� ʽ�ζ��ܣ�
�ڵζ�ʱ��������ƿ�мӷ�̪��Ϊָʾ��������εζ����۲쵽 ʱΪ�ζ��յ㡣
���й����ݼ�¼���£�
| �ζ���� | ����Һ�����(mL) | ���������Һ�����(mL) | |
| ��ʼ���� �ζ�ǰ | �յ���� | ||
| 1 | 20.00 | 0.50 | 20.40 |
| 2 | 20.00 | 6��00 | 26��10 |
| 3 | 20.00 | 4��00 | 24��00 |
���ݴ�����NaOH��Һ��Ũ��Ϊ mol��L-1���ռ���Ʒ�Ĵ���Ϊ ����������λ��Ч���֣�
���Է������¸��������ʵ�����Ŀ���Ӱ�죬�á�ƫ�ߡ��p��ƫ�͡�����Ӱ�족����գ�
����������ˮ��ϴ��ƿ�����ʹ�ⶨ�Ľ�� ��
�����ڵζ������в�����������Һ������ƿ�⣬���ʹ�ⶨ��� ��
�����ռ���ָʾ���ֲ�����ɫ�б仯��ֹͣ�ζ������ʹ�ⶨ��� ��
�ܶ���ʱ�����ζ�ǰ���Ӷ������ζ������Ӷ��������ʹ�ⶨ��� ��
����A��B��C��D��E����������ˮ��ǿ����ʣ������������������(�������Ӳ��ظ�)��
| ������ | H+��NH4+��Mg2+��Ba2+��Al3+ |
| ������ | OH-��Cl-��HCO3-��NO3-��SO42- |
��C��Һ
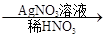 ��ɫ��������ش��������⣺
��ɫ��������ش��������⣺��1��д���������ʵĻ�ѧʽ��A______________��B______________��
��2��д����C��Һ
 ��ɫ�������йط�Ӧ�����ӷ���ʽ____________________________��
��ɫ�������йط�Ӧ�����ӷ���ʽ____________________________����3��D��E���������б���һ����_______________��д�������������ʵ���Һ�μӵ�B��Һ�з�Ӧ�����ӷ���ʽ___________________________________________________________________��
��4���������ʵ��ȷ��C����һ��δ֪�����ʲô���ʡ�(ֻ����A��E��ѡ������Լ�)
| ʵ�鲽�� | Ԥ������ͽ��� |
| ȡ����C����Һ���Թ��У� �� �� | Ԥ������ͽ���1�� �� �� Ԥ������ͽ���2�� �� �� |