��Ŀ����
����Ŀ����ͼ����������̽�����������ʣ�ʵ��ʱ��NaOH�����ϵμ���Ũ��ˮ����������һ������������档�±��ж�ʵ������������������Ľ��;���ȷ����
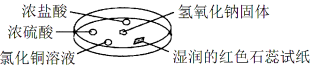
ѡ�� | ʵ �� �� �� | �� �� |
A | Ũ���ḽ���������� | NH3��HCl��Ӧ������NH4Cl���� |
B | Ũ���ḽ������������ | NH3��Ũ���������Ӧ |
C | �Ȼ�ͭ��Һ���ɫ���� | ��ɫ���ǵijɷ���Cu(OH)2 |
D | ʪ��ĺ�ɫʯ����ֽ���� | NH3��һ�ֿ����Լ� |
���𰸡�A
��������
���������A����NaOH�����ϵμ���Ũ��ˮ���������������Ũ���ᷴӦ�����Ȼ�泥���Ӧ�������а������ɣ���ȷ��B����NaOH�����ϵμ���Ũ��ˮ���������������Ũ���ᷢ����Ӧ��������泥�����C����NaOH�����ϵμ���Ũ��ˮ��������������������Ȼ�ͭ��Һ������Ӧ����������ͭ��ɫ����������D������ʹʪ��ĺ�ɫʯ����ֽ������ԭ������ˮ��Ӧ����NH3H2O����������OH-���ӣ���Һ�ʼ��ԣ�������Ϊ�ǵ���ʣ������ڼ����

��ϰ��ϵ�д�
�����Ŀ