��Ŀ����
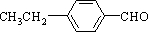
3����֪��R-CH=CH-O-R�䣨ϩ�ѣ�$\stackrel{H_{2}O/H+}{��}$ R-CH2CHO+R��OH��A����Է���������Mr��Ϊ176��������̼��ԭ����Ŀ��Ϊ3��4������C�ڼ��ȵ��������ܹ���������Һ������D��ͬʱ���ɹ�����������������Ӧ������A��صķ�Ӧ��ͼ��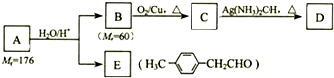
��ش��������⣺
��1��A�Ľṹ��ʽΪ
 ��
����2��д��B��C��Ӧ�Ļ�ѧ����ʽ��2CH3CH2CH2OH+O2$��_{��}^{Cu}$2CH3CH2CHO+2H2O��
��3��ͬʱ��������������E��ͬ���칹����3�֣�
�����ڷ���ȩ���ڱ����������ֲ�ͬ��������ԭ�ӣ�
���� �������и����ʵ�ת����ϵ��B�ܷ���������Ӧ����C��C�ڼ��ȵ��������ܹ���������Һ������D����BΪ����CΪȩ����B����Է�������Ϊ60����BΪCH3CH2CH2OH��B��C������������Ӧ��CΪCH3CH2CHO��C��DΪ������Ӧ����DΪCH3CH2COONH4������ϩ����A��̼��ԭ����Ŀ��Ϊ3��4������Է�������Ϊ176���ṹB��E�Ľṹ����Ϣ��֪��AΪ ���ݴ˽��
���ݴ˽��
��� �⣺�������и����ʵ�ת����ϵ��B�ܷ���������Ӧ����C��C�ڼ��ȵ��������ܹ���������Һ������D����BΪ����CΪȩ����B����Է�������Ϊ60����BΪCH3CH2CH2OH��B��C������������Ӧ��CΪCH3CH2CHO��C��DΪ������Ӧ����DΪCH3CH2COONH4������ϩ����A��̼��ԭ����Ŀ��Ϊ3��4������Է�������Ϊ176���ṹB��E�Ľṹ����Ϣ��֪��AΪ ��
��
��1��������������֪��AΪ ��
��
�ʴ�Ϊ�� ��
��
��2��B��C����CH3CH2CH2OH�Ĵ���������Ӧ�Ļ�ѧ����ʽΪ��2CH3CH2CH2OH+O2 $��_{��}^{Cu}$ 2CH3CH2CHO+2H2O��
�ʴ�Ϊ��2CH3CH2CH2OH+O2 $��_{��}^{Cu}$ 2CH3CH2CHO+2H2O��
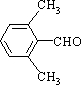
��3��E��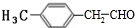 ����ͬ���칹����������������ڷ���ȩ�������к��б�����-CHO���ڱ����������ֲ�ͬ��������ԭ�ӣ��ɶԳƿ�֪������ֻ������Hԭ�ӣ���ȡ����Ϊ�һ���-CHO����������-CHO�����Է�������E��ͬ���칹��Ϊ
����ͬ���칹����������������ڷ���ȩ�������к��б�����-CHO���ڱ����������ֲ�ͬ��������ԭ�ӣ��ɶԳƿ�֪������ֻ������Hԭ�ӣ���ȡ����Ϊ�һ���-CHO����������-CHO�����Է�������E��ͬ���칹��Ϊ ��
�� ��
�� ����3�֣�
����3�֣�
�ʴ�Ϊ��3��
���� ���⿼���л��ƶϣ������л��ϳ��ۺ������⣬ȫ�濼�����л�����ʽ�ͽṹ��ʽ���Ƶ�����ѧ����ʽ����д��ͬ���칹����ж�����д���л���Ϣ��������Ӧ�ã�ȫ�濼����ѧ��˼ά��������������ͽ��������������Ѷ��еȣ�

| pH | 6.5��8.5 |
| Ca2+��Mg2+ | ��0.004 5mol•L-1 |
| ϸ������ | ��100��/mL |
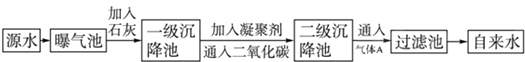
��1��Դˮ�к�Ca2+��Mg2+��HCO3-��Cl-�ȣ�������ʯ�Һ�����Ca��OH��2�������������ɸ��ֽⷴӦ��д������һ�����ӷ���ʽ��Ca2++HCO-3+OH-=CaCO3��+H2O��?Mg2++2HCO-3+2Ca2++4OH-=Mg��OH��2��+2CaCO3��+2H2O��Mg2++2OH-=Mg��OH��2������
��2�����ۼ���ȥ������������Ĺ��̢ۣ�����ţ���FeSO4•7H2O�dz��õ����ۼ�������ˮ���������ɽ�״Fe��OH��3������
��ֻ���������̡� ��ֻ�ǻ�ѧ���� ���������ͻ�ѧ����
��3��ͨ�������̼��Ŀ���dz�ȥ��ȥCa2+�͵���PHֵ��
��4������A��������ǿ���������������ǻ�������A��ˮ��Ӧ�IJ������Cl2+H2O=H++Cl-+HClO�ԣ�
��5�����������Т٢ۿ�����Ϊ����A�Ĵ���Ʒ��
��Ca��ClO��2��������NH3��Һ�� ��K2FeO4�� ����SO2��

| A�� | �¶���ͬ����t1�棩ʱ��KNO3��Һ�����ʵ�������������NaCl��Һ�����ʵ��������� | |
| B�� | t1��ʱ��KNO3��NaCl������Һ�����ʵ����ʵ���Ũ����� | |
| C�� | �á��ܽ�����������ȹ��ˡ��������ᴿ��������KNO3���ʵ�NaCl���� | |
| D�� | ��50g KNO3������Һ��t2�潵��t1�棬������������С��0.5��b-a��g |
| A�� | Na+��Fe2+��NO3-��Cl- | B�� | Ca2+��K+��Cl-��NO3- | ||
| C�� | Al3+��K+��OH-��NO3- | D�� | Na+��Ca2+��SiO32-��Cl- |
| A�� | �ܢ٢ۢ� | B�� | �ܢ٢ۢ� | C�� | �ܢۢܢ� | D�� | �٢ڢܢ� |
| A�� | 2 | B�� | 1.7 | C�� | 12 | D�� | 12.3 |
| A�� | CaO+H2O�TCa��OH��2��H��0 | B�� | 2CH3OH��l��+3O2��g���T2CO2��g��+4H2O��l����H��0 | ||
| C�� | 4Fe��OH��2��s��+2H2O��l��+O2��g���T4 Fe��OH��3��s����H��0 | D�� | 2H2��g��+O2��g���T2H2O��l����H��0 |
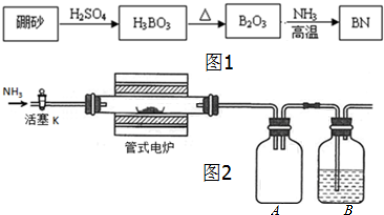
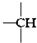 ��
��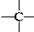 ������Ŀ�ֱ���a��b��c��d��ʾ�����������������⣩�д��ڵĹ�ϵ�����ۣ�
������Ŀ�ֱ���a��b��c��d��ʾ�����������������⣩�д��ڵĹ�ϵ�����ۣ�