��Ŀ����
ȡδ֪��Ȼ������A 66.50g����Ԫ�ط�������ǿ����Һ���ȣ��ڱ�״�����ռ���N2 5.60L�����赪ȫ��ת��������֪������1�������к���һ����ԭ�ӣ���ȡ2.66g A����ˮ�����Һ����0.80mol/L NaOH��Һ�ζ���ȥ50.00mLǡ���кͣ�����
��1��������A��Ħ������
��2��������A�����к��Ȼ��ĸ���
��3������A�Ļ�ѧʽ����д��A���жԳƽṹM������ļ�ʽ��
��1��������A��Ħ������
��2��������A�����к��Ȼ��ĸ���
��3������A�Ļ�ѧʽ����д��A���жԳƽṹM������ļ�ʽ��
���㣺�й��л������ʽȷ���ļ���
ר�⣺�������������ȼ�չ���
��������1������n=
���㵪�������ʵ���������NԪ���غ����n��-NH2��������ȷ������������ʵ������ٸ���M=
����ð������Ħ��������
��2������2.66g����������ʵ���������NaOH�����ʵ������谱������x��-COOH����R��COOH��x����xNaOH���ݴ˼����Ȼ���Ŀ��
��3���谱��������к���n��CH2�����ݺ��еİ������Ȼ���Ŀȷ��������ķ���ʽ����ʽ�������Է�������ȷ������ʽ����д���жԳƽṹM������ṹ��ʽ��
| V |
| Vm |
| m |
| n |
��2������2.66g����������ʵ���������NaOH�����ʵ������谱������x��-COOH����R��COOH��x����xNaOH���ݴ˼����Ȼ���Ŀ��
��3���谱��������к���n��CH2�����ݺ��еİ������Ȼ���Ŀȷ��������ķ���ʽ����ʽ�������Է�������ȷ������ʽ����д���жԳƽṹM������ṹ��ʽ��
���
�⣺��1��m��A��=66.5g��n��-NH2��=2n��N2��=2��
=0.5 mol
������A��Ħ������Ϊ��M��A��=66.5g��0.5mol=133g/mol
�𣺰�����A��Ħ������Ϊ133g/mol��
��2��2.26gA�����ʵ���Ϊ��2.66g��133g/mol=0.02mmol
�����������Ƶ����ʵ���=0.8mol/L��0.05L=0.04mol
�谱������x��-COOH����
R��COOH��x����x NaOH
1 x
0.02mol 0.04mol
x=
=2����A��2��-COOH��
�𣺰�����A�����к���2���Ȼ���
��3���谱����A�Ľṹ��ʽΪ��HOOC-CH��NH2��-��CH2��n-COOH����
��������A��ѧʽΪ��C3+nH5+2nO4N��
��12����3+n��+5+2n+16��4+14=133
���n=1��
��������A��ѧʽΪ��C4H7O4N��A���жԳƽṹM������Ľṹ��ʽ��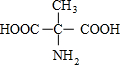 ��
��
�𣺰�����A��ѧʽΪ��C4H7O4N��A���жԳƽṹM������Ľṹ��ʽ��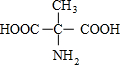 ��
��
| 5.6L |
| 22.4L/mol |
������A��Ħ������Ϊ��M��A��=66.5g��0.5mol=133g/mol
�𣺰�����A��Ħ������Ϊ133g/mol��
��2��2.26gA�����ʵ���Ϊ��2.66g��133g/mol=0.02mmol
�����������Ƶ����ʵ���=0.8mol/L��0.05L=0.04mol
�谱������x��-COOH����
R��COOH��x����x NaOH
1 x
0.02mol 0.04mol
x=
| 0.04mol��1 |
| 0.02mol |
�𣺰�����A�����к���2���Ȼ���
��3���谱����A�Ľṹ��ʽΪ��HOOC-CH��NH2��-��CH2��n-COOH����
��������A��ѧʽΪ��C3+nH5+2nO4N��
��12����3+n��+5+2n+16��4+14=133
���n=1��
��������A��ѧʽΪ��C4H7O4N��A���жԳƽṹM������Ľṹ��ʽ��
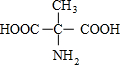 ��
���𣺰�����A��ѧʽΪ��C4H7O4N��A���жԳƽṹM������Ľṹ��ʽ��
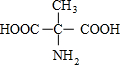 ��
��
���������⿼���л����ƶϣ��ѶȲ���ȷ���Ȼ���Ŀ�ǹؼ���ע�����հ���������ʣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
�����л����У������³���̬���ǣ�������
| A��CH3Cl |
| B��CH2Cl2 |
| C��CCl4 |
| D��CH3COOH |
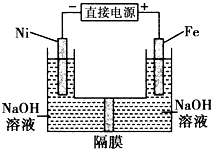 ������������Դ���������й㷺��;��������Ni���������缫���ŨNaOH��Һ�Ʊ���������Na2FeO4��װ����ͼ��ʾ�������ƶϺ������ǣ�������
������������Դ���������й㷺��;��������Ni���������缫���ŨNaOH��Һ�Ʊ���������Na2FeO4��װ����ͼ��ʾ�������ƶϺ������ǣ�������| A�������������缫��ӦΪFe-6e-+4H2O�TFeO42-+8H+ |
| B�����缫�ϵĵ缫��ӦΪ2H2O+2e-�TH2��+2OH- |
| C������ĤΪ�����ӽ���Ĥ����OH-���������ƶ� |
| D�����ʱ������pH���͡�������pH���ߣ�������ҺpH���� |
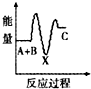
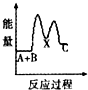
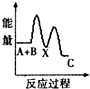
 H2O2����Ҫ��������������ˮ��Һ������������ɱ����Ư�ȣ���ش������й����⣮
H2O2����Ҫ��������������ˮ��Һ������������ɱ����Ư�ȣ���ش������й����⣮