��Ŀ����
����Ŀ���мס�����λͬѧ��������ԭ��ط�Ӧ�������Ļ��˳�������˾���þƬ����Ƭ���缫������ͬѧ���缫����6 mol��L��1��H2SO4��Һ������ͬѧ���缫����6 mol��L��1��NaOH��Һ������ͼ��ʾ��
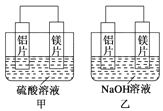
��1��д�����������ĵ缫��Ӧʽ___________________��
��2�������ܷ�Ӧ�����ӷ���ʽ��________________________��
��3�����������ͬѧ����Ϊ������ԭ��صĵ缫����������ǽ������ɸ������ϵĽ���Ӧ�ȹ����������ϵĽ������á�������жϳ� ��Ը�ǿ�����һ��жϳ�_____________��Ը�ǿ��(��дԪ�ط���)
��4���ɴ�ʵ��ó������н���������ȷ����______________��
A������ԭ��ط�Ӧ�жϽ������˳��ʱӦע��ѡ����ʵĽ���
B��þ�Ľ����Բ�һ�������Ľ�����ǿ
C����ʵ��˵���������˳����ѹ�ʱ��û��ʵ�ü�ֵ��
D����ʵ��˵����ѧ�о������ӡ���Ӧ������Ӱ��ϴ���Ӧ��������������
���𰸡���1��2H����2e��==H2����2��2Al��2OH����2H2O==2AlO2-��3H2��
��3��Mg Al��4��AD
��������
�����������1������þ��ʧ������������Al��������������þ����������Ӧ�������������ӷ�����ԭ��Ӧ��������ӦΪ2H++2e-=H2����������ӦΪMg-2e-=Mg2+��
��2���ҳ�������ʧ������������þ����������������ʧ���ӷ���������Ӧ������ܷ�ӦΪ��2Al��2OH����2H2O==2AlO2-��3H2����
��3������þ���������������������������������Ľ���������ǿ�жϣ�����þ���ǿ�����������ǿ��
��4��A�����ݼס����е缫��Ӧʽ֪��ԭ�����������������Һ�йأ�����ȷ��B��þ�Ľ����Դ���������ʧ�������׳̶���������Һ�йأ��ʴ���C����ʵ����о����ʵ�������ʵ�ü�ֵ���ʴ���D����ʵ��˵����ѧ�о������ӣ���Ӧ�������йأ��缫������ͬ�䷴Ӧ������ͬ��������ﲻͬ������Ӧ��������������������ȷ����ѡAD��

����Ŀ��ʵ����Ҫ0��80 mol��L-1NaOH��Һ475 mL��0��40 mol��L-1������Һ500 mL��������������Һ����������ش��������⣺
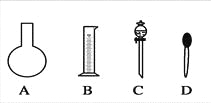
��1����ͼ��ʾ��������������Һ�϶�����Ҫ����________������ţ�������������Һ�����õ��IJ���������________�����������ƣ���
��2�����в����У�����������ƿʵ�ֵ���______________������ţ���
A������һ�����ȷŨ�ȵı���Һ |
B����ȡһ�������Һ�� |
C����������ƿ������µ����������Һ�� |
D��ȷϡ��ijһŨ�ȵ���Һ |
E��������Һ
F�����������ܽ��������
��3�����ݼ�����������ƽ��ȡNaOH������Ϊ___________g����ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ��_________��������������������������С��������ͬ��0��8 mol��L-1��������ʱ������������ˮ����������ƿ�⣬��������ҺŨ��__________0.8 mol��L-1��
��4�����ݼ����֪��������������Ϊ98 %���ܶ�Ϊ1��84 mol��L-1��Ũ��������Ϊ__________mL������������һλС���������ʵ������10 mL��15 mL��20 mL��50 mL��Ͳ��ѡ��___________mL��Ͳ��á�