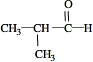
��Ŀ����
5��V��W��X��Y��Z�������ֶ�����Ԫ���е����ֻ�������ɵ�5�ֻ��������W��X��Z��������Ԫ����ɣ�X�ǵ�������ЧӦ����Ҫ���壬Z����Ȼ������Ҫ�ɷ֣�Y��W���������ᷴӦ��������ǿ����Һ��Ӧ������5�ֻ������漰������Ԫ�ص�ԭ������֮�͵���28��V��һ�ֽ���Ԫ�غ����ַǽ���Ԫ����ɣ���ԭ�Ӹ�����Ϊ1��3��9������ԭ����������������н���Ԫ�ص�ԭ������������֮��ķ�Ӧ��ϵ��ͼ��
��1��д��W���ʵ�һ����;�����ͻ���ϻ�ұ����������
��2��д��V������NaOH��Һ��Ӧ�Ļ�ѧ����ʽAl��CH3��3+NaOH+H2O=NaAlO2+3CH4����
��3����������Xͨ��ij�����ʵ�ˮ��Һ�п�������Y���÷�Ӧ�����ӷ���ʽΪAlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
��4��4g Z��ȫȼ������X��Һ̬ˮ�ų�222.5kJ����������д����ʾZȼ���ȵ��Ȼ�ѧ����ʽCH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890KJ/mol��
��5����200mL 1.5mol•L-1 NaOH��Һ��ͨ���״����4.48L X���壬��ȫ��Ӧ��������Һ�У���������Ũ���ɴ�С��˳����c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
��6��Y��һ���������ʣ����ܶȻ�����Ϊ1.25��10-33����0.01mol YͶ��1LijŨ�ȵ������У�ΪʹY��ȫ�ܽ�õ���������Һ���������Ũ������ӦΪ3.02��10-2mol/L������仯���Բ��ƣ����������λ��Ч���֣���
���� X�ǵ�������ЧӦ����Ҫ���壬ӦΪCO2��Z����Ȼ������Ҫ�ɷ֣�ӦΪCH4��Y��W���������ᷴӦ��������ǿ����Һ��Ӧ��ӦΪ���Ի������ת����ϵ��֪YΪAl��OH��3��WΪAl2O3����V�к���C��Al��HԪ�أ�ԭ�Ӹ�����Ϊ1��3��9��ӦΪAl��CH3��3��
��1��WΪAl2O3���۵�ߣ������ڸ����ͻ���ϻ�ұ����������
��2��VAl��CH3��3��������NaOH��Һ��Ӧ���ɺͼ��飻
��3����������NaAlO2ͨ��ij�����ʵ�ˮ��Һ�п�������Al��OH��3��Ӧͨ�������̼��
��4��4g����Ϊ0.25mol��0.25mol������ȫȼ������Һ̬ˮʱ�ų�222.5KJ������1mol���鷴Ӧȼ�շ�Ӧ����$\frac{222.5}{0.25}$=890KJ��
��5��4.48L CO2ͨ�뵽200mL 1.5mol/LNaOH��Һ�У�������̼�����ʵ���=$\frac{4.48L}{22.4L/mol}$=0.2mol���������Ƶ����ʵ���1.5mol/L��0.2L=0.3mol��
��1��n��NaOH����n��CO2����2ʱ����������̼���ƺ�̼�����ƣ�����ʽΪ2CO2+3NaOH=Na2CO3+NaHCO3+H2O���Դ��жϣ�
��6����0.01mol Al��OH��3Ͷ��1LijŨ�ȵ������У���������Ҫ0.03mol��ΪʹAl��OH��3��ȫ�ܽ�õ���������Һ����Ӧ��c��OH-��=$\root{3}{\frac{1.25��1{0}^{-33}}{0.01}}$mol/L=0.5��10-10mol/L����c��H+��=2��10-4mol/L��
��� �⣺X�ǵ�������ЧӦ����Ҫ���壬ӦΪCO2��Z����Ȼ������Ҫ�ɷ֣�ӦΪCH4��Y��W���������ᷴӦ��������ǿ����Һ��Ӧ��ӦΪ���Ի������ת����ϵ��֪YΪAl��OH��3��WΪAl2O3����V�к���C��Al��HԪ�أ�ԭ�Ӹ�����Ϊ1��3��9��ӦΪAl��CH3��3��
��1��WΪAl2O3���۵�ߣ������ڸ����ͻ���ϻ�ұ�����������ʴ�Ϊ�������ͻ���ϻ�ұ����������
��2��VAl��CH3��3��������NaOH��Һ��Ӧ���ɺͼ��飬��Ӧ�Ļ�ѧ����ʽAl��CH3��3+NaOH+H2O=NaAlO2+3CH4����
�ʴ�Ϊ��Al��CH3��3+NaOH+H2O=NaAlO2+3CH4����
��3����������NaAlO2ͨ��ij�����ʵ�ˮ��Һ�п�������Al��OH��3��Ӧͨ�������̼����Ӧ�����ӷ���ʽΪAlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
�ʴ�Ϊ��AlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
��4��4g����Ϊ0.25mol��0.25mol������ȫȼ������Һ̬ˮʱ�ų�222.5KJ������1mol���鷴Ӧȼ�շ�Ӧ����$\frac{222.5}{0.25}$=890KJ����Ӧ���Ȼ�ѧ����ʽΪ��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890KJ/mol��
�ʴ�Ϊ��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890KJ/mol��
��5��4.48L CO2ͨ�뵽200mL 1.5mol/LNaOH��Һ�У�������̼�����ʵ���=$\frac{4.48L}{22.4L/mol}$=0.2mol���������Ƶ����ʵ���1.5mol/L��0.2L=0.3mol��
��1��n��NaOH����n��CO2����2ʱ����������̼���ƺ�̼�����ƣ�
���ݶ�����̼�������������ʵ���֮��Ĺ�ϵ�÷���ʽΪ2CO2+3NaOH=Na2CO3+NaHCO3+H2O�����ݷ���ʽ֪��̼���ƺ�̼�����Ƶ����ʵ�����ȣ�̼������Ӻ�̼���������ˮ���ʹ��Һ�ʼ��ԣ���c��OH-����c��H+����̼������ӵ�ˮ����������̼��������ӣ�����c��HCO3-����c��CO32-���������Ӳ�ˮ�⣬����Ũ�������������Ũ�ȴ�С˳����c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
��6��Al��OH��3��һ���������ʣ����ܶȻ�����Ϊ1.25��10-33����0.01mol Al��OH��3Ͷ��1LijŨ�ȵ������У���������Ҫ0.03mol��ΪʹAl��OH��3��ȫ�ܽ�õ���������Һ����Ӧ��c��OH-��=$\root{3}{\frac{1.25��1{0}^{-33}}{0.01}}$mol/L=0.5��10-10mol/L����c��H+��=2��10-4mol/L��������Ҫ�����Ũ��Ϊ3.02��10-2mol/L��
�ʴ�Ϊ��3.02��10-2mol/L��
���� ���⿼��������ƶϣ�Ϊ��Ƶ����ͳ������ͣ�������ѧ���ķ��������������Ŀ��飬��Ҫ���ճ������ʵ����ʡ���;�ͷ�Ӧ������Ϊ��������Ŀ�Ĺؼ����Ѷ��еȣ�

| A�� | ����Ƭ��ϡ���ᷴӦ������ʱ���ɸ���98%��Ũ����ӿ������������� | |
| B�� | 100 mL 2 mol•L-1�������пƬ��Ӧ�������������Ȼ�����Һ����Ӧ���ʲ��� | |
| C�� | ����β����NO��CO���Ի�����Ӧ����N2��CO2����Сѹǿ��Ӧ���ʼ��� | |
| D�� | SO2�Ĵ�������һ�����ȷ�Ӧ�����������¶ȣ���Ӧ���ʼ��� |
| ʵ����� | ʵ��Ŀ�� | |
| A�� | ���� NO2 ���ܱղ�����ֱ�������䡢��ˮ�� | �о��¶ȶԻ�ѧƽ���ƶ���Ӱ�� |
| B�� | ��ʢ�� 1mL ��������Һ���Թ��еμ� NaCl ��Һ���������г������������еμ� Na2S ��Һ | ˵��һ�ֳ�����ת��Ϊ��һ�� �ܽ�ȸ�С�ij��� |
| C�� | ���Ӻ�ˮ����Һ�У�������Ũ̼������Һ | �Ƚϱ�����̼������� |
| D�� | ��2֧�Թ��м���ͬ���ͬŨ��H2C2O4��Һ���ٷֱ����ͬ�����ͬŨ�ȵ�����KMnO4�� Һ | ������ɫʱ�����о�Ũ�ȶԷ� Ӧ���ʵ�Ӱ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ����ɫ��Һ�У�NH4+��Fe2+��SO42-��CO32- | |
| B�� | �ں��д���Ba2+ ����Һ�У�NH4+��Na+��Cl-��CO32- | |
| C�� | ��ǿ������Һ�У�Na+��Cl-��K+��SO42- | |
| D�� | ��ǿ������Һ�У�K+��Fe2+��Cl-��HCO3- |
| A�� | 25��ʱ��NH4Cl��NH3��H2O�Ļ��Һ������Ũ�ȿ���ΪC��NH4+����C��Cl-����C��OH-����C��H+�� | |
| B�� | ��0.1mol/L��CuSO4��Һ�м�����������ˮ����Һ���Լ�����Cu2+ˮ��̶Ƚ��� | |
| C�� | 25��ʱ��PH=12��NaOH��Һ��PH=12��CH3COONa��Һ��PH=2�����ᣬ������Һ��ˮ�ĵ���̶���ͬ | |
| D�� | 25��ʱ����ϡ��ˮ��ͨ��NH3����Һ��C��OH-����С |


 R1COOH+R2COOH ��R1��R2����������
R1COOH+R2COOH ��R1��R2����������