��Ŀ����
16����ʽ̼��þ������ˮ����;�㷺����Ҫ��������Ʒ������������ǿ����ĥ�Ժ�ǿ�ȣ�Ҳ�����������Ϳ�ϵ����Ӽ���Ҳ���������ࡢҽҩ�ͻ�ױƷ�ȹ�ҵ����ˮ��þʯ����Ҫ�ɷ�ΪMgCl2•6H2O��Ϊԭ��������ʽ̼��þ����Ҫ�������£�
��1��Ԥ������������Mg��OH��2�������ɣ���֪������Ksp��Mg��OH��2��=1.8��10-11����ʾMg��OH��2�����ܽ�ƽ��ķ���ʽΪMg��OH��2��s��?Mg2+��aq��+2OH-��aq����Mg��OH��2�ﵽ�����ﵽ�����ܽ�ƽ��ʱ��Һ��pH10.5����֪��lg36��1.5����
��2����֪��������Ka1��H2CO3��=4.4��10-7��Ka2��H2CO3��=4.7��10-11��Kb��NH3•H2O��=1.8��10-5����NH4HCO3��Һ�Լ� �ԣ�c��NH$\stackrel{+}{4}$������ c��HCO$\overline{3}$����ѡ����ڡ�����С�ڡ��������ڡ���������Һ�����غ����ʽΪc��NH4+��+c��NH3•H2O��=c��CO32-��+c��HCO3-��+c��H2CO3����
��3�����������е���ҺŨ���ᾧ��������Ҫ�������ʵĻ�ѧʽΪNH4Cl��
��4���������ռ�ʽ̼��þ�õ�MgO��ȡ��ʽ̼��þ����4.84g���������������أ��õ�����2.00g�ͱ�״����CO2 0.896L�����ʽ̼��þ�Ļ�ѧʽΪMg��OH��2•4MgCO3•4H2O����Mg5��OH��2��CO3��4•4H2O����д���Ȼ�þ������̼�������ˮ�����ɼ�ʽ̼��þ�����ӷ���ʽ5Mg2++4HCO3-+6NH3+6H2O=Mg��OH��2•4MgCO3•4H2O��+6NH4+��
���� ˮ��þʯ����Ҫ�ɷ�ΪMgCl2•6H2O��Ϊԭ��������ʽ̼��þ����Ҫ���̣���ˮ�ܽ⣬ͨ�백����������������ˮ���õ����Եİ�ˮ���ټ���̼����泥��ٽ�����ˮ����Խ�þ���ӳ����������γɼ�ʽ̼��þ���������˼��ɵõ�Ŀ�껯���
��1��Mg��OH��2�����ܽ�����þ���Ӻ����������ӣ�����Ksp=[Mg2+][OH-]2������������Ũ�ȣ�����ȷ����Һ��pHֵ��
��2��Ka1��H2CO3��Kb��NH3•H2O��������笠����ӵ�ˮ��̶�С��̼��������ӣ����ݵ�Ԫ�غ�̼Ԫ���غ��д�������غ�ʽ��
��3�����������ж�������Һ�ijɷ��к��е����ӣ�ȷ����Һ�е��������ش�
��4��̼��þ����д��������ı�ʾ��ʽ����xMgO•yCO2•zH2O��Ȼ���������ȷ���������������������ȷ������ʽ������Ԫ���غ�͵���غ���д���ӷ���ʽ��
��� �⣺ˮ��þʯ����Ҫ�ɷ�ΪMgCl2•6H2O��Ϊԭ��������ʽ̼��þ����Ҫ���̣���ˮ�ܽ⣬ͨ�백����������������ˮ���õ����Եİ�ˮ���ټ���̼����泥��ٽ�����ˮ����Խ�þ���ӳ����������γɼ�ʽ̼��þ���������˼��ɵõ�Ŀ�껯���
��1��Mg��OH��2�����ܽ�����þ���Ӻ����������ӣ��ܽ�����ӷ���ʽΪMg��OH��2��s��?Mg2+��aq��+2OH-��aq��������Ksp=[Mg2+][OH-]2��֪��[OH-]=$\root{3}{2K{\;}_{sp}}$=$\root{3}{2��1.8��10{\;}^{-11}}$=$\root{3}{36}$��10-4mol•L-1��[H+]=$\frac{1}{\root{3}{36}}$��10-10mol•L-1��������Һ��pH=10+$\frac{1}{3}$lg36=10.5��
�ʴ�Ϊ��Mg��OH��2��s��?Mg2+��aq��+2OH-��aq����10.5��
��2��Ka1��H2CO3��Kb��NH3•H2O��������笠����ӵ�ˮ��̶�С��̼��������ӣ�������Һ��c��NH4+����c��HCO3-������Һ�ʼ��ԣ����ݵ�Ԫ�غ�̼Ԫ���غ��֪�����غ�ʽΪc��NH4+��+c��NH3•H2O��=c��CO32-��+c��HCO3-��+c��H2CO3����
�ʴ�Ϊ������ڣ�c��NH4+��+c��NH3•H2O��=c��CO32-��+c��HCO3-��+c��H2CO3����
��3�����������ж�������Һ�ijɷ��к��е������������Ӻ�笠����ӣ������õ���Һ�к�NH4Cl��
�ʴ�Ϊ��NH4Cl��
��4��̼��þ����д��������ı�ʾ��ʽ����xMgO•yH2O•zCO2���������⣺��ʽ̼��þ4.84g���������������أ��õ�����2.00g�ͱ�״����CO20.896L����n��CO2��=0.896L/22.4L•mol-1=4.00��10-2 mol��n��MgO��=2.00g/40g•mo l-1=5.00��10-2 mol��n��H2O��=$\frac{4.84g-4.00��10{\;}^{-2}mol��44g/mol-2.00g}{18g/mol}$=5.00��10-2 mol��n��MgO����n��CO2����n��H2O��=5.00��10-2��4.00��10-2��5.00��10-2=5��4��5����x��y��z=5��4��5�����Լ�ʽ̼��þ�Ļ�ѧʽΪ��Mg��OH��2•4 MgCO3•4H2O����Mg5��OH��2��CO3��4•4H2O�������Ȼ�þ������̼�������ˮ�����ɼ�ʽ̼��þ�����ӷ���ʽΪ5Mg2++4HCO3-+6NH3+6H2O=Mg��OH��2•4MgCO3•5H2O��+6NH4+��
�ʴ�Ϊ��Mg��OH��2•4 MgCO3•4H2O����Mg5��OH��2��CO3��4•4H2O����5Mg2++4HCO3-+6NH3+6H2O=Mg��OH��2•4MgCO3•4H2O��+6NH4+��
���� ���⿼��þ�Ļ�����������Լ��Ʊ�ʵ�鷽������ƣ���Ŀ�Ѷ��еȣ�����ע�⣨4���������غ�ĽǶȷ��������л�ѧʽ�ļ��㣬Ϊ�״��㣮

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| A�� | �ü�ͼװ�õ�⾫���� | |
| B�� | ����ͼװ���Ʊ� Fe��OH��2 | |
| C�� | �ñ�ͼװ�ÿ��Ƶý����� | |
| D�� | �ö�ͼװ����֤ NaHCO3 �� Na2CO3�����ȶ��� |
| A�� | ��ԭ�ԣ�X-��Y- | |
| B�� | ��X-��Y-��Z-��W- �� Z- �Ļ�ԭ����ǿ | |
| C�� | �����ԣ�Z2��W2 | |
| D�� | ��Ӧ2Z-+Y2=2Y-+Z2���Է��� |
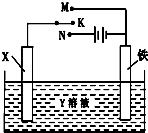
| A�� | ��XΪп����YΪNaCl��Һ������K����M�����ɼ������ĸ�ʴ�����ַ�����Ϊ�������������������� | |
| B�� | ��XΪͭ����YΪ����ͭ��Һ������K����M����ͭ�����������ӣ���ʱ���·�еĵ�����ͭ�缫�ƶ� | |
| C�� | ��XΪ̼����YΪNaCl��Һ������K����N�����ɼ������ĸ�ʴ����Һ�е������������缫�ƶ� | |
| D�� | ��XΪͭ����YΪ����ͭ��Һ������K����N�����������������ӣ���Һ��ͭ����Ũ�Ƚ���С |
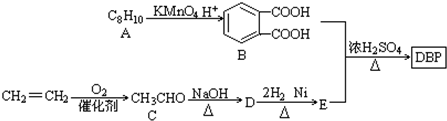
 $\stackrel{KMnO_{2}H+}{��}$
$\stackrel{KMnO_{2}H+}{��}$
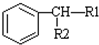
 ��-R1��-R2��ʾ��ԭ�ӻ�������
��-R1��-R2��ʾ��ԭ�ӻ�������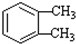 ��D��E�ķ�Ӧ���ͼӳɣ���ԭ����Ӧ��
��D��E�ķ�Ӧ���ͼӳɣ���ԭ����Ӧ��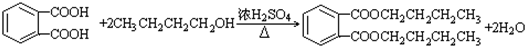 ��
��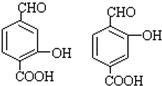 ��
��

 ��������ʹ����KMnO4��Һ��ɫ���������Ӧ�������˸����ʵĻ�ԭ�ԣ�������ԡ���ԭ�ԡ���
��������ʹ����KMnO4��Һ��ɫ���������Ӧ�������˸����ʵĻ�ԭ�ԣ�������ԡ���ԭ�ԡ���