��Ŀ����
2�����л�ѧ����ı�����Ӧ��ȷ���ǣ�������| A�� | ̼�����Ƶĵ��뷽��ʽ��NaHCO3�TNa++H++CO${\;}_{3}^{2-}$ | |
| B�� | ��ͭ����������Ȼ�ͭ��Һ�����ӷ���ʽ��Cu2++2Cl-$\frac{\underline{\;���\;}}{\;}$Cu+Cl2�� | |
| C�� | ����ˮ������ӷ���ʽ��S2-+2H2O�TH2S+2OH- | |
| D�� | ��TiCl4�Ʊ�TiO2�Ļ�ѧ����ʽ��TiCl4+��x+2��H2O��������?TiO2•x H2O��+4HCl |
���� A��̼���������Ϊ��Ԫ�������ʽ�����ӣ�Ӧ������ѧʽ��
B��ͭ��������Ϊ���Ե缫���缫�������뷴Ӧ��
C����Ԫ��������ӷֲ�ˮ�⣬�Ե�һ��Ϊ����
D�����Ȼ���Ϊǿ�������Σ�ˮ��Һ��ˮ������TiO2•x H2O�����ȷֽ�����TiO2��
��� �⣺A��̼�����Ƶĵ��뷽��ʽ��NaHCO3�TNa++HCO3-����A����
B����ͭ����������Ȼ�ͭ��Һ�����ӷ���ʽ��Cu+2H2O$\frac{\underline{\;���\;}}{\;}$Cu��OH��2��+Cl2��+H2������B����
C�������ӷ�����ˮ�⣬��һ��ˮ��������������ӣ�ˮ�����ӷ���ʽΪ��S2-+H2O?HS-+OH-����C����
D�����Ȼ���Ϊǿ�������Σ�ˮ��Һ��ˮ������TiO2•x H2O�����ȷֽ�����TiO2����D��ȷ��
��ѡ��D��
���� ���⿼�������ӷ�Ӧ����ʽ�����жϣ���ȷ���ʵ����ʡ�����ˮ����ǽ���ؼ���ע���Ԫ��������ӵ�ˮ�ⷽʽ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ
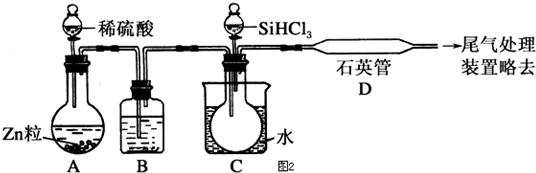
13���¶�ΪTʱ����2.0L�����ܱ������г���1.0mol PCl5����ӦPCl5��g��?PCl3��g��+Cl2��g������һ��ʱ���ﵽƽ�⣮��Ӧ�����вⶨ�IJ������ݼ��±���
����˵����ȷ���ǣ�������
| t/s | 0 | 50 | 150 | 250 | 350 |
| n��PCl3��/mol | 0 | 0.16 | 0.19 | 0.20 | 0.20 |
| A�� | ��Ӧ��ǰ50 s ��ƽ������v��PCl3��=0.0032 mol•L-1•s-1 | |
| B�� | ���������������䣬�����¶ȣ�ƽ��ʱc��PCl3��=0.11 mol•L-1����Ӧ��H��0 | |
| C�� | ��ͬ�¶��£���ʼʱ�������г���1.0 mol PCl5��0.20 mol PCl3 ��0.20 mol Cl2����Ӧ�ﵽƽ��ǰ v��������v���棩 | |
| D�� | ��ͬ�¶��£���ʼʱ�������г���2.0 mol PCl3��2.0 mol Cl2���ﵽƽ��ʱ��PCl3 ��ת����С��80% |
17��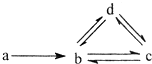 ���и�����������������ͼ��ʾת����ϵ���ǣ�ͼ�м�ͷ��ʾһ��ת������������
���и�����������������ͼ��ʾת����ϵ���ǣ�ͼ�м�ͷ��ʾһ��ת������������
 ���и�����������������ͼ��ʾת����ϵ���ǣ�ͼ�м�ͷ��ʾһ��ת������������
���и�����������������ͼ��ʾת����ϵ���ǣ�ͼ�м�ͷ��ʾһ��ת������������| a | b | c | d | |
| �� | Si | SiO2 | H2SiO3 | Na2SiO3 |
| �� | N2 | NO | NO2 | HNO3 |
| �� | Cu | CuO | Cu��OH��2 | CuSO4 |
| �� | Na | NaCl | Na2CO3 | NaHCO3 |
| A�� | �٢� | B�� | �ڢ� | C�� | �ۢ� | D�� | �� |
7��������ʵ������ƽ���ƶ�ԭ�����͵��ǣ�������
| A�� | �Ӵ�����ʹN2��H2��һ��������ת��ΪNH3 | |
| B�� | ����ѹǿ��������SO2��O2��Ӧ����SO3 | |
| C�� | ��H2��I2��g����HI��g��������ɵ�ƽ����ϵ��ѹ����ɫ���� | |
| D�� | ����ɫ����ˮ���պ���ɫ��dz |
11�������й����ʼ����ʵ�������ȷ���ǣ�������
| ѡ�� | ʵ �� �� �� �� �� �� | ʵ �� �� �� |
| A | ��ij��Һ�м������ᱵ��Һ���а�ɫ�������ɣ�����ϡ���ᣬ����δ��ʧ | ����Һ��һ������SO42- |
| B | ��ij����ͨ��Ʒ����Һ�У�Ʒ����Һ��ɫ | ������һ����SO2 |
| C | ��ij��Һ�м���2��KSCN��Һ����Һ���Ժ�ɫ��������Һ�е��뼸�����Ƶ���ˮ����Һ��Ϊ��ɫ | ����Һ��һ������Fe2+ |
| D | ������ij���ʵ���Һ�μӵ����Ƶ�������Һ�У�ˮԡ���Ⱥ����������� | ������һ������ȩ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |