��Ŀ����
ijѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ�������ָʾ��������д���пհף�
��1���ñ�������ζ������NaOH��Һʱ����������ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע�� ��ֱ��������һ���������Һ�� ɫ��Ϊ ���� Ϊֹ��
��2�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���________
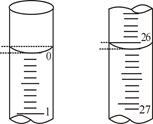
��3�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ����Ӧ������������Ϊ________ mL��

��4��ijѧ������3��ʵ��ֱ��¼�й��������±���
�����ϱ��������NaOH��Һ�����ʵ���Ũ��Ϊ ��
��1���ñ�������ζ������NaOH��Һʱ����������ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע�� ��ֱ��������һ���������Һ�� ɫ��Ϊ ���� Ϊֹ��
��2�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���________
| A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע������� |
| B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и��� |
| C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ |
| D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��� |

��4��ijѧ������3��ʵ��ֱ��¼�й��������±���
| �ζ� ���� | ����NaOH��Һ�����/mL | 0��100 mol/L��������/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25��00 | 0��20 | 20��22 | |
| �ڶ��� | 25��00 | 0��56 | 24��54 | |
| ������ | 25��00 | 0��42 | 20��40 | |
�����ϱ��������NaOH��Һ�����ʵ���Ũ��Ϊ ��
��ƿ����Һ��ɫ�ı仯��1�֣� ��ɫ��1�֣� ��ɫ��1�֣�����Ӳ���ɫ��1�֣�
��2��D��1�֣� ��3��20��00mL��1�֣� ��4��0��0800 mol/L��2�֣�
��2��D��1�֣� ��3��20��00mL��1�֣� ��4��0��0800 mol/L��2�֣�
�����������1������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯���ζ��յ�ʱ��Һ��ɫ�ɻ�ɫͻ��Ϊ��ɫ���Ұ�����ڲ���ɫ����2��A����ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���������Һ����Һ��Ũ��ƫС�����V������ƫ����c�����⣩=
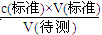 ��֪���ⶨc��NaOH��ƫ��A�����ϡ�B���ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и������Һ�����ʵ������䣬��V��������Ӱ�죬����c�����⣩=
��֪���ⶨc��NaOH��ƫ��A�����ϡ�B���ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и������Һ�����ʵ������䣬��V��������Ӱ�죬����c�����⣩=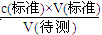 ��֪���ⶨc��NaOH����Ӱ�죬��B�����ϣ�Cѡ����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ�����V������ƫ����c�����⣩=
��֪���ⶨc��NaOH����Ӱ�죬��B�����ϣ�Cѡ����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ�����V������ƫ����c�����⣩=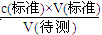 ��֪���ⶨc��NaOH��ƫ��C�����ϣ�Dѡ���ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������V������ƫС������c�����⣩=
��֪���ⶨc��NaOH��ƫ��C�����ϣ�Dѡ���ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������V������ƫС������c�����⣩=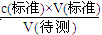 ��֪���ⶨc��NaOH��ƫ�ͣ���D���ϣ���3����ʼ����Ϊ0��10mL���յ����Ϊ20��10mL��������Һ�����Ϊ20��00mL����4���������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V�����ᣩ=20��00mL��
��֪���ⶨc��NaOH��ƫ�ͣ���D���ϣ���3����ʼ����Ϊ0��10mL���յ����Ϊ20��10mL��������Һ�����Ϊ20��00mL����4���������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V�����ᣩ=20��00mL��HCl +NaOH = NaCl +H2O
0��0200L��0��1000mol/L 0��025L��C��NaOH��
��C��NaOH��=
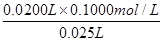 =0��0800 mol/L
=0��0800 mol/L
��ϰ��ϵ�д�
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
�����Ŀ

 H����OH�����ڲ�ͬ�¶������ӻ�ΪKW(25��)��1.0��10��14��
H����OH�����ڲ�ͬ�¶������ӻ�ΪKW(25��)��1.0��10��14��