��Ŀ����
Ϊ�ⶨijƷ��ϴ������(����)������ijѧ��������֪���ʵ���Ũ�ȵ�NaOH��Һ���ⶨ��ϴ��(����)�����ʵ���Ũ��ʱ��ѡ���̪��ָʾ��������д���пհף�
��1����ʽ�ζ��ܵ�ʹ�÷�������ȷ������Ⱥ�˳��Ϊ����ѡ����ţ���
A��������������Һ��ϴ��������������������Һ
B���ų�����������Һ���еζ�
C��������ʼ����
D����©����ˮϴ2-3��
��2���ñ���NaOH��Һ�ζ����������ʱ,���ֿ��Ƽ�ʽ�ζ��ܵIJ���������ҡ����ƿ���۾�Ӧע�� ��
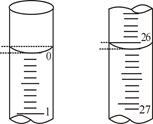
��3�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ������ͼ��ʾ��������NaOH��Һ�����Ϊ mL��
��4��ijѧ����������ʵ��ֱ��¼�й��������±���
��ѡ�����к��������ݼ����ϴ������(����)�����ʵ���Ũ�ȣ�
c(HCl)�� ��
��5���������в���������ϴ������(����)��Ũ�ȵ�Ӱ�죺���ƫ����ƫС�� ����Ӱ�족��
A����ȡ����Һʱ����ʼ���Ӷ����� ���Ӷ������� ��
B������ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ���� ��
��1����ʽ�ζ��ܵ�ʹ�÷�������ȷ������Ⱥ�˳��Ϊ����ѡ����ţ���
A��������������Һ��ϴ��������������������Һ
B���ų�����������Һ���еζ�
C��������ʼ����
D����©����ˮϴ2-3��
��2���ñ���NaOH��Һ�ζ����������ʱ,���ֿ��Ƽ�ʽ�ζ��ܵIJ���������ҡ����ƿ���۾�Ӧע�� ��

��3�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ������ͼ��ʾ��������NaOH��Һ�����Ϊ mL��
��4��ijѧ����������ʵ��ֱ��¼�й��������±���
| �ζ����� | ������������/mL | 0.1000 mol/LNaOH��Һ�����/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 2.00 | 28.15 | 26.15 |
| �ڶ��� | 25.00 | 1.50 | 29.50 | 28.00 |
| ������ | 25.00 | 0.20 | 26.55 | 26.35 |
��ѡ�����к��������ݼ����ϴ������(����)�����ʵ���Ũ�ȣ�
c(HCl)�� ��
��5���������в���������ϴ������(����)��Ũ�ȵ�Ӱ�죺���ƫ����ƫС�� ����Ӱ�족��
A����ȡ����Һʱ����ʼ���Ӷ����� ���Ӷ������� ��
B������ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ���� ��
(1) DACB��
��2����ƿ����Һ��ɫ�ı仯��
��3��26.10mL��
��4�� c(HCl)��0.105 mol/L��
��5��ƫ��ƫ��
��2����ƿ����Һ��ɫ�ı仯��
��3��26.10mL��
��4�� c(HCl)��0.105 mol/L��
��5��ƫ��ƫ��
�����������1���������裺�����������ϴ������װҺ��������ʼ�������к͵ζ����յ��жϡ����㡢���ݴ�����
��2���ζ�ʱ�����ֿ��ƻ��������ְ�˳ʱ��ҡ����ƿ���۾��۲���ƿ����Һ��ɫ�ı仯��
��3���۲����ʱҪƽ�ӣ�
��4��c(HCl)��0.1000*��26.35+26.15��/2/25.00="0.105" mol/L��
��5��ȡ����Һʱ�����Ӻ��ӣ���ʹ����Һ�Ķ���ƫС ��ʹ����ϴ������(����)��Ũ��ƫ�����ζ����������ݣ���ʹ��Һ�������ƫ�Ӷ�ʹ����ϴ������(����)��Ũ��ƫ��

��ϰ��ϵ�д�
 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�
�����Ŀ


