��Ŀ����
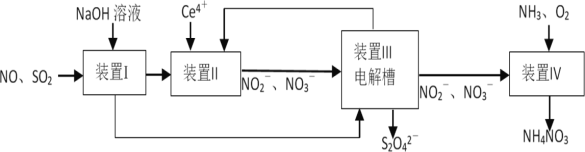
����Ŀ��Ϊ������������������������չ�ҵβ��SO2��NO��ͬʱ���������������(Na2S2O4����ᾧˮ�����ֳƱ��շ�)��NH4NO3��Ʒ���Ա�������Ϊ����
����˵���������
A.S2O![]() �мȴ��ڷǼ��Լ��ִ��ڼ��Լ�
�мȴ��ڷǼ��Լ��ִ��ڼ��Լ�
B.װ��I������������SO2��װ��II������������NO
C.���շۿ�ͨ��װ���������������Ʊ���Ce4���������������ص�װ��IIѭ��ʹ��
D.����װ��IV��1L 2molL��1NO![]() ��������Ҫ��״����22.4LO2
��������Ҫ��״����22.4LO2
���𰸡�C
��������
�������̷�����֪��װ�����м���NaOH��Һ���ɷ�����ӦSO2+OH-=![]() ����SO2��װ�����м���Ce4+�����������£�NO��Ce4+����������ԭ��Ӧ����
����SO2��װ�����м���Ce4+�����������£�NO��Ce4+����������ԭ��Ӧ����![]() ��
��![]() ��Ce4+����ԭΪCe3+��װ����(����)������������ӦCe3+-e-=Ce4+��Ce4���������������ص�װ��IIѭ��ʹ�ã�����������Ӧ2
��Ce4+����ԭΪCe3+��װ����(����)������������ӦCe3+-e-=Ce4+��Ce4���������������ص�װ��IIѭ��ʹ�ã�����������Ӧ2![]() +2H++2e-=
+2H++2e-=![]() +2H2O�������õ����շ�Na2S2O4��װ������
+2H2O�������õ����շ�Na2S2O4��װ������![]() ��O2����Ϊ
��O2����Ϊ![]() ��
��![]() ��NH3�õ�NH4NO3���ݴ˷������
��NH3�õ�NH4NO3���ݴ˷������
A��![]() ��Sԭ����Oԭ���γɼ��Լ���Sԭ����Sԭ���γɷǼ��Լ���Aѡ����ȷ��
��Sԭ����Oԭ���γɼ��Լ���Sԭ����Sԭ���γɷǼ��Լ���Aѡ����ȷ��
B����������������֪��װ�����м���NaOH��Һ���ɷ�����ӦSO2+OH-=![]() ����SO2��װ�����м���Ce4+�����������£�NO��Ce4+����������ԭ��Ӧ����
����SO2��װ�����м���Ce4+�����������£�NO��Ce4+����������ԭ��Ӧ����![]() ��
��![]() ��������NO��Bѡ����ȷ��
��������NO��Bѡ����ȷ��
C��װ����(����)������������ӦCe3+-e-=Ce4+��Ce4���������������ص�װ��IIѭ��ʹ�ã�Cѡ�����
D��װ������![]() ��O2����Ϊ
��O2����Ϊ![]() ��NԪ�ػ��ϼ���+3��������+5�ۣ�O�Ļ��ϼ���0�۽�����-2�ۣ�����װ��IV��1L 2molL-1(2mol)NO
��NԪ�ػ��ϼ���+3��������+5�ۣ�O�Ļ��ϼ���0�۽�����-2�ۣ�����װ��IV��1L 2molL-1(2mol)NO![]() ����ת��4mol���ӣ�����1molO2������Ҫ��״����22.4LO2��Dѡ����ȷ��
����ת��4mol���ӣ�����1molO2������Ҫ��״����22.4LO2��Dѡ����ȷ��
��ѡC��

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�