题目内容
常温下,下列各溶液的叙述中正确的是
[c(Na+)-c(F一)]<[c(K+)-c(CH3COO一)]
| A.pH=7的醋酸钠和醋酸混合液中:c(Na+)=c(CH3COO-) |
| B.0.1mol/L的醋酸的pH=a,0.01mol/L的醋酸的pH=b,则a+1>b |
| C.0.1mol/L的醋酸钠溶液20mL与0.1mol/L盐酸10mL混合后溶液显酸性 c (CH3COO-)>c (Cl-)>c (H+)>c (CH3COOH) |
| D.已知酸性HF>CH3COOH,pH相等的NaF与CH3COOK溶液中, |
AB
A中醋酸钠考虑水解平衡、醋酸考虑电离平衡,则溶液中存在的离子有:Na+、H+、CH3COO-、OH-,由电荷守恒,得:c(Na+)+ c(H+)=c(CH3COO-)+c(OH-)
pH=7,则c(H+)=c(OH-),故c(Na+)=c(CH3COO-),A正确;
B、“0.1mol/L的醋酸的pH=a”,则c(H+)=10-amol.L-1,醋酸浓度由0.1mol/L变到0.01mol/L(相当于稀释了10倍),若醋酸的电离平衡不移动,则c(H+)=10-(a+1)mol.L-1,但溶液越稀,电离程度越大,所以实际上c(H+)>10-(a+1)mol.L-1,即PH(b =)<a+1,故B正确;
C、CH3COONa+HCl= CH3COOH+NaCl,反应后变为等浓度的CH3COONa、CH3COOH、NaCl的混合物,
醋酸钠:CH3COONa=CH3COO-+Na+,CH3COO-+ H2O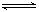 CH3COOH+ OH-
CH3COOH+ OH-
醋酸: CH3COOH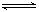 CH3COO-+ H+,NaCl: NaCl= Na++ Cl-
CH3COO-+ H+,NaCl: NaCl= Na++ Cl-
利用守恒思想:C(CH3COO-)+C(CH3COOH)=( 0.1mol/L×0.02L)/0.03L="0.2/3" mol/L
“显酸性”说明醋酸的电离程度大于醋酸根的水解程度,则c (CH3COO-)>c (CH3COOH),
即:c (CH3COO-)>0.1/3 mol.L-1、c (CH3COOH)<0.1/3 mol.L-1,而c (Cl-) ="0.1/3" mol.L-1,
故c (CH3COO-)>c (Cl-) >c (CH3COOH) >c (H+)(醋酸的电离程度很小),故C错误;
D、NaF溶液:NaF=Na++F-,F-+ H2O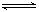 HF+ OH-,
HF+ OH-,
电荷守恒:c(Na+)+ c(H+)=c(F-)+c(OH-),
则c(Na+)-c(F一)= c(OH-)- c(H+)
CH3COOK溶液:CH3COOK=CH3COO-+K+,CH3COO-+ H2O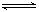 CH3COOH+ OH-,
CH3COOH+ OH-,
电荷守恒:c(K+)+ c(H+)=c(CH3COO-)+c(OH-)
则c(K+)-c(CH3COO一)= c(OH-)- c(H+)
两溶液“pH相等”即c(H+)相等、在相同温度下,则c(OH-)也相等,
故[c(Na+)-c(F一)]=[c(K+)-c(CH3COO一)],D错误;
pH=7,则c(H+)=c(OH-),故c(Na+)=c(CH3COO-),A正确;
B、“0.1mol/L的醋酸的pH=a”,则c(H+)=10-amol.L-1,醋酸浓度由0.1mol/L变到0.01mol/L(相当于稀释了10倍),若醋酸的电离平衡不移动,则c(H+)=10-(a+1)mol.L-1,但溶液越稀,电离程度越大,所以实际上c(H+)>10-(a+1)mol.L-1,即PH(b =)<a+1,故B正确;
C、CH3COONa+HCl= CH3COOH+NaCl,反应后变为等浓度的CH3COONa、CH3COOH、NaCl的混合物,
醋酸钠:CH3COONa=CH3COO-+Na+,CH3COO-+ H2O
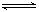 CH3COOH+ OH-
CH3COOH+ OH-醋酸: CH3COOH
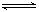 CH3COO-+ H+,NaCl: NaCl= Na++ Cl-
CH3COO-+ H+,NaCl: NaCl= Na++ Cl-利用守恒思想:C(CH3COO-)+C(CH3COOH)=( 0.1mol/L×0.02L)/0.03L="0.2/3" mol/L
“显酸性”说明醋酸的电离程度大于醋酸根的水解程度,则c (CH3COO-)>c (CH3COOH),
即:c (CH3COO-)>0.1/3 mol.L-1、c (CH3COOH)<0.1/3 mol.L-1,而c (Cl-) ="0.1/3" mol.L-1,
故c (CH3COO-)>c (Cl-) >c (CH3COOH) >c (H+)(醋酸的电离程度很小),故C错误;
D、NaF溶液:NaF=Na++F-,F-+ H2O
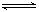 HF+ OH-,
HF+ OH-,电荷守恒:c(Na+)+ c(H+)=c(F-)+c(OH-),
则c(Na+)-c(F一)= c(OH-)- c(H+)
CH3COOK溶液:CH3COOK=CH3COO-+K+,CH3COO-+ H2O
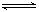 CH3COOH+ OH-,
CH3COOH+ OH-,电荷守恒:c(K+)+ c(H+)=c(CH3COO-)+c(OH-)
则c(K+)-c(CH3COO一)= c(OH-)- c(H+)
两溶液“pH相等”即c(H+)相等、在相同温度下,则c(OH-)也相等,
故[c(Na+)-c(F一)]=[c(K+)-c(CH3COO一)],D错误;

练习册系列答案
 名师金手指领衔课时系列答案
名师金手指领衔课时系列答案
相关题目
 大小顺序是③>②>①
大小顺序是③>②>①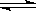 H++SO42—
H++SO42—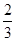 c(Na+)= c(HCO3—)+ c(CO32—)+ c(H2CO3)
c(Na+)= c(HCO3—)+ c(CO32—)+ c(H2CO3)