��Ŀ����
3��ij��ѧ��ȤС��Ҫ����к��ȵIJⶨ����1��ʵ�����ϱ����ձ�����С�����ձ�������ĭ���ϡ���Ͳ����ĭ���ϰ塢��ͷ�ιܡ����β�������0.5mol•L-1���ᡢ0.55mol•L-1NaOH��Һ����ȱ�ٵ�ʵ�鲣����Ʒ���¶ȼƣ�
��2����ʵ��װ���Ͽ����ձ���������������ĭ�������Ǽ���ʵ������е�������ʧ
��3�����ձ����粻��Ӳֽ�壬����õ��к�����ֵƫС���ƫ����ƫС��������Ӱ�족��
��4��ʵ���и���50mL 0.50mol/L�Ĵ����50mL 0.55mol/L��NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�����������ȣ����ȡ�����ȡ�������������Ϊ�ᡢ����кͷ�Ӧ�ų����������ᡢ��������йأ�
���� ��1�������к��Ȳⶨ��ʵ�鲽��ѡ����Ҫ��������Ȼ���жϻ�ȱ�ٵ�������
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�����
��3������Ӳֽ�壬����һ��������ɢʧ��
��4����Ӧ�ų����������������Լ�������Ķ����йأ�
��� �⣺��1���к��ȵIJⶨ�����У���Ҫ����Ͳ��ȡ����Һ������Һ���������Ҫʹ���¶ȼƲ����¶ȣ����Ի�ȱ���¶ȼƣ�
�ʴ�Ϊ���¶ȼƣ�
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�������С�ձ�֮��������ֽ���������ǣ����¡����ȣ�����ʵ������е�������ʧ��
�ʴ�Ϊ������ʵ������е�������ʧ��
��3�����ձ����粻��Ӳֽ�壬����һ��������ɢʧ����õ��к�����ֵ�����С��
�ʴ�Ϊ��ƫС��
��4����Ӧ�ų����������������Լ�������Ķ����йأ�����50mL 0.50mol/L�Ĵ����50mL 0.55mol/L��NaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ��
�ʴ�Ϊ������ȣ���Ϊ�ᡢ����кͷ�Ӧ�ų����������ᡢ��������йأ�
���� ���⿼��ѧ���й��к��ȵIJⶨ��ע������ʵ��IJⶨԭ�����ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
17����Ҫ����0.01mol/L��KMnO4��Һ�����в�������������ҺŨ��ƫ�ߵ��ǣ�������
| A�� | ����ʱ��ʹ�������룬�������ҩƷ�ŵߵ��� | |
| B�� | ����ʱ��������ƿ�̶��� | |
| C�� | ԭ����ƿϴ����û�и��� | |
| D�� | ҡ�Ⱥ��Һ���½����ټ�ˮ���̶��� |
14�����ĺϳ�������Ҫ�Ļ�������֮һ��
��ҵ�Ϻϰ��õ�H2�ж�����ȡ�ķ�����
���ý�̿��ˮ��Ӧ��C��s��+H2O��g��$\frac{\underline{\;����\;}}{\;}$CO��g��+H2��g����
������Ȼ����ˮ������Ӧ��CH4��g��+H2O��g��$\frac{\underline{\;����\;}}{����}$CO��g��+3H2��g��
��֪�йط�Ӧ�������仯��ͼ��ʾ���ҷ����ڵķ�Ӧֻ���ڸ����·����������з�Ӧ�ġ�H=��a+3b-c��KJ/mol��

����3��1L���ܱ������У�ͬ�¶��¡�ʹ����ͬ�����ֱ���з�Ӧ��
3H2��g��+N2��g��$?_{����}^{���¡���ѹ}$2NH3��g��������ͬ��ʽ���뷴Ӧ����ֺ��¡����ݣ���Ӧ�ﵽƽ��ʱ�й�����Ϊ��
��1��������˵���÷�Ӧ�Ѵﵽƽ��״̬����c��
a��������N2��H2��NH3��Ũ��֮��Ϊ1��3��2 b��v��N2����=3v��H2����
c��������ѹǿ���ֲ��� d�����������ܶȱ��ֲ���
��2���������дﵽƽ�������ʱ��t��5min�����������������=������
��3�����дӷ�Ӧ��ʼ��ƽ��ʱN2��ƽ����Ӧ����0.2mol/��L��min������ע����λ����
��4�������ϱ����ݣ����й�ϵ��ȷ����c��
a.2c1=3mol/L b����1=��2 c.2��1=��2
��5�����¶��£��������У��÷�Ӧ��ƽ�ⳣ��K=$\frac{4}{81}$���÷�����ʾ����mol/L��-2��
��1������������N2��H2Ϊ��Ӧ�������A��ϡ����Ϊ�������Һ��������������ṩ���ܣ����̵ܹ�������ȼ�ϵ�أ�װ����ͼ1��ʾ��
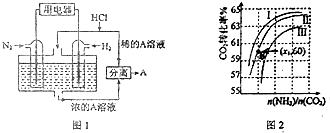
��������ĵ缫��Ӧʽ��N2+8H++6e-=2NH4+��A���Ȼ�泥�
��2���ð��ϳ����صķ�ӦΪ2NH3��g��+CO2��g��?CO��NH2��2��s��+H2O��g������ҵ����ʱ��ԭ��������ˮ������ͼ2��ʾCO2��ת�����백̼��$\frac{n��N{H}_{3}��}{n��C{O}_{2}��}$��ˮ̼��$\frac{n��{H}_{2}O��}{n��C{O}_{2}��}$�ı仯��ϵ��
�����ߢ��Ӧ��ˮ̼������Ǣ�
�ڲ��B�㰱��ת����Ϊ40%����x13��
��ҵ�Ϻϰ��õ�H2�ж�����ȡ�ķ�����
���ý�̿��ˮ��Ӧ��C��s��+H2O��g��$\frac{\underline{\;����\;}}{\;}$CO��g��+H2��g����
������Ȼ����ˮ������Ӧ��CH4��g��+H2O��g��$\frac{\underline{\;����\;}}{����}$CO��g��+3H2��g��
��֪�йط�Ӧ�������仯��ͼ��ʾ���ҷ����ڵķ�Ӧֻ���ڸ����·����������з�Ӧ�ġ�H=��a+3b-c��KJ/mol��

����3��1L���ܱ������У�ͬ�¶��¡�ʹ����ͬ�����ֱ���з�Ӧ��
3H2��g��+N2��g��$?_{����}^{���¡���ѹ}$2NH3��g��������ͬ��ʽ���뷴Ӧ����ֺ��¡����ݣ���Ӧ�ﵽƽ��ʱ�й�����Ϊ��
| ���� | �� | �� | �� |
| ��Ӧ��Ͷ���� | 3molH2��2molN2 | 6molH2��4molN2 | 2molNH3 |
| �ﵽƽ���ʱ�䣨min�� | T | 5 | 8 |
| ƽ��ʱN2��Ũ�ȣ�mol•L-1�� | C1 | 3 | |
| N2��������� | ��1 | ��2 | ��3 |
| ��������ܶȣ�g•L-1�� | ��1 | ��2 |
a��������N2��H2��NH3��Ũ��֮��Ϊ1��3��2 b��v��N2����=3v��H2����
c��������ѹǿ���ֲ��� d�����������ܶȱ��ֲ���
��2���������дﵽƽ�������ʱ��t��5min�����������������=������
��3�����дӷ�Ӧ��ʼ��ƽ��ʱN2��ƽ����Ӧ����0.2mol/��L��min������ע����λ����
��4�������ϱ����ݣ����й�ϵ��ȷ����c��
a.2c1=3mol/L b����1=��2 c.2��1=��2
��5�����¶��£��������У��÷�Ӧ��ƽ�ⳣ��K=$\frac{4}{81}$���÷�����ʾ����mol/L��-2��
��1������������N2��H2Ϊ��Ӧ�������A��ϡ����Ϊ�������Һ��������������ṩ���ܣ����̵ܹ�������ȼ�ϵ�أ�װ����ͼ1��ʾ��

��������ĵ缫��Ӧʽ��N2+8H++6e-=2NH4+��A���Ȼ�泥�
��2���ð��ϳ����صķ�ӦΪ2NH3��g��+CO2��g��?CO��NH2��2��s��+H2O��g������ҵ����ʱ��ԭ��������ˮ������ͼ2��ʾCO2��ת�����백̼��$\frac{n��N{H}_{3}��}{n��C{O}_{2}��}$��ˮ̼��$\frac{n��{H}_{2}O��}{n��C{O}_{2}��}$�ı仯��ϵ��
�����ߢ��Ӧ��ˮ̼������Ǣ�
�ڲ��B�㰱��ת����Ϊ40%����x13��
11����һ���¶��£����ݻ��㶨���ܱ������з�����Ӧ��2A��s��+2B��g��?C��g��+D��g���������������������仯ʱ����˵���÷�Ӧ�Ѵﵽƽ��״̬���ǣ�������
�ٻ������ƽ����Է������� ������������ѹǿ �ۻ�����������ʵ��� ��C���ʵ���Ũ�ȣ�
�ٻ������ƽ����Է������� ������������ѹǿ �ۻ�����������ʵ��� ��C���ʵ���Ũ�ȣ�
| A�� | ֻ�Тڢ� | B�� | �٢� | C�� | �ڢۢ� | D�� | ֻ�Т� |
18�������йظ���ж�����������ȷ���ǣ�������
| A�� | CO2����ˮ���ܵ��磬��CO2Ϊ����� | |
| B�� | H216O��D216O��H218O��D218O��Ϊͬ�������� | |
| C�� | NaCl��Һ��CH3COONH4��Һ�������ԣ�������Һ��ˮ�ĵ���̶���ͬ | |
| D�� | ��Һ�뽺�屾������Ϊ��ɢ����ֱ���Ĵ�С��ͬ |
8���������ʵ��������������ó��Ľ��۴�����ǣ�������
| ѡ �� | ʵ����� | ʵ������ | ���� |
| A | ȡ���õ�Na2O2��ĩ�������еμӹ��������� | ������ɫ���� | Na2O2û����ȫ���� |
| B | ����������ˮ����FeCl2��NaI�Ļ����Һ�У��ٵμ�CCl4�������ã����ϲ���Һ�еμ�KSCN��Һ�������ϲ���Һ�еμ���ˮ | ʵ������1�� �ϲ���Һ����죬�²���Һ���Ϻ�ɫ ʵ������2�� �ϲ���Һ��� | �����ԣ�Br2��Fe3+��I2 |
| C | ��һƬ�������ھƾ������������� | �����ۻ��������� | ��������������Al2O3��Ĥ����Al2O3�۵����Al�� |
| D | ȡ���õ��̷���FeSO4•7H2O������ˮ������KSCN��Һ | ��Һ��Ϊ��ɫ | �̷����ֻ�ȫ�������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
15�����������У�����ֱ�����ڼ��ȵ��ǣ�������
| A�� | �ձ� | B�� | ���� | C�� | ������ | D�� | �Թ� |
13����������NaCl��Һ����������ȷ���ǣ�������
| A�� | ת��ʱ��Ҫ�ò��������� | |
| B�� | ������ƽ��ȡ58.50��ʳ�Σ���ˮ���Ƴ�1����Һ��NaCl��ҺŨ��Ϊ1mol•L-1 | |
| C�� | �ܽ�ʳ�ε��ձ�Ҫϴ��2��3�β���ϴ��Һת�Ƶ�����ƿ�� | |
| D�� | ����ɼ����ܽ� |