��Ŀ����
����Ŀ����֪A��B��C��D���ֶ�����Ԫ�صĺ˵������������Aԭ��s�����������p�����������������Cԭ�ӵ�L�ܲ��������ԳɶԵ��ӣ�C��Dͬ���塣 E��F�ǵ�������Ԫ�أ���Eλ�����ڱ���ds���� Fԭ��������33�ֲ�ͬ�˶�״̬�ĵ��ӡ�����������Ϣ����Ӧ��Ԫ�ط�����գ�
��1��E+��������Ų�ʽΪ ��FC43�������Ŀռ乹��Ϊ �����以Ϊ�ȵ������һ���л�����Ϊ ���ѧʽ����
��2��BԪ���������ڵ�һ����������Ԫ���� ����Ԫ�ط�������
��3��D��������Ԫ������������Ӧ��ˮ�����У�������ǿ���� ���ѧʽ�����ܵ����A������B��D��E�ĵ����γɵľ�����Ƚϣ��۵��ɸߵ��͵�����˳���� ���ѧʽ����
��4����֪EDC4��Һ�е��백����������H2N��CH2��COONa�����ɵõ������A����ṹ��ͼ��ʾ��
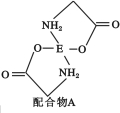
�� �����A��̼ԭ�ӵĹ���ӻ�����Ϊ ��
�� 1 mol������������H2N��CH2��COONa��������������ĿΪ ��
��5��������F2C3�����ڱ궨δ֪Ũ�ȵ�����KMnO4��Һ����Ӧ����F����ۺ����ᣬ�÷�Ӧ�����ӷ���ʽ�� ��
���𰸡���1��1s22s22p63s23p63d10��[Ar]3d10 ��2�������������� ��1������CCl4 ��1���� ��2��Ne ��1����
��3��HClO4 ��1������ C��Cu��S��N2 ��2����
��4���� SP2��SP3 ����1�֣���2���� �� 8NA��8��6.02��1023 ��2����
��5��5As2O3 + 4MnO4- + 9H2O + 12H+ == 10H3AsO4 + 4Mn2+ ��2����
��������
�����������������֪A��B��C��D���ֶ�����Ԫ�صĺ˵��������������Aԭ��s�����������p�����������������Aԭ�ӵĺ�������Ų�ʽΪ1s22s22p2����AΪ̼Ԫ�أ�Cԭ�ӵ�L�ܲ��������ԳɶԵ��ӣ�Cԭ�ӵĺ�������Ų�ʽΪ1s22s22p4����CΪ��Ԫ�أ�BΪ��Ԫ�أ�C��Dͬ��������DΪ��Ԫ���� E��F�ǵ�������Ԫ�أ���Eλ�����ڱ���ds�������γ�E+����EΪͭԪ�أ�Fԭ��������33�ֲ�ͬ�˶�״̬�ĵ�������FΪ��Ԫ����
��1��EΪͭԪ�أ�ͭΪ29��Ԫ�أ����ݹ���ԭ��д��Cu+��������Ų�ʽΪ1s22s22p63s23p63d10��[Ar]3d10��AsO43����������ԭ��Asԭ�Ӳ�ȡsp3�ӻ���û�й¶Ե��ӣ��ռ乹��Ϊ���������������以Ϊ�ȵ������һ���л�����ΪCCl4��
��2��BΪ��Ԫ����λ�ڵڶ����ڣ�ͬ������������Ԫ��ԭ�ӵĵ�һ�����������ʵ�Ԫ���������ڵ�һ����������Ԫ����Ne��
��3��DΪ��Ԫ�أ�λ�ڵ������ڣ���������Ԫ������������Ӧ��ˮ�����У�������ǿ����HClO4��ʯīΪ����;��壬�۵���ߣ�ͭΪ�������壬�۵��֮�����N2Ϊ���Ӿ��壬N2�ڳ�����Ϊ���壬�۵���ͣ��۵��ɸߵ��͵�����˳����C��Cu��S��N2��
��4���� ���������A�Ľṹ�ж�A��̼ԭ�ӵĹ���ӻ�����ΪSP2��SP3��
�����������ƵĽṹ�ж�1 mol������������H2N��CH2��COONa��������������ĿΪ8NA��8��6.02��1023��
��5��As2O3������KMnO4��Һ��Ӧ��������H3AsO4�����û��ϼ����������ԭ���غ㡢����غ���ƽ���÷�Ӧ�����ӷ���ʽ��5As2O3 + 4MnO4- + 9H2O + 12H+ == 10H3AsO4 + 4Mn2+��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ���±��������ֶ�����Ԫ�ص������Ϣ��
Ԫ�� | �����Ϣ |
A | ���¡���ѹ�£��䵥������������壬������Ϊ�������������ȼ�� |
B | ��ҵ��ͨ������Һ̬��������䵥�ʣ���ij��ͬ���������DZ�������ر���������Ҫ���� |
C | ֲ��������Ҫ��֮һ�������γɶ��������ijЩ���������ɹ⻯ѧ��Ⱦ |
D | �������䵥��Ϊ����ɫ��ĩ״���壬�������ۻ����õ�����������ȼ�գ���������������ɫ���� |
E | �������䵥��Ϊ����ɫ���壬�䵥��ˮ��Һ��Ư�ס�ɱ������ |
�밴Ҫ��ش��������⣺
��1��DԪ�غ�������Ų� ��
��2��A��B��Ԫ���γɵĺ��зǼ��Լ��Ļ�����Ľṹʽ ��A��B��Ԫ���γɵľ�����ͬ�������������У��ѧʽ����______________��_____________��
��3��C��A�γɵ�ijһ�������ܺ�C��B�γɵ���һ��ɫ�������������ֻ����������ԭ�Ӹ����Ƚ�Ϊ1��2��һ������������Ƽ������߷�����Ӧ���������ʣ�д��������ѧ��Ӧ����ʽ�� ��
��4��һ�������£���ˮ��Һ��1 mol E����EO��x��1,2,3,4����������kJ����Դ�С��ͼ��ʾ��n�� �������ӷ����������ӷ�Ӧy��x��m���Ȼ�ѧ����ʽΪ ��

��5��Na2DB3ˮ��Һ�и�����Ũ�ȵĴ�С˳���� �������ӷ��ţ���