��Ŀ����
����Ŀ����ԭ�������ĵ��������ֶ�����Ԫ��(����ĸx�ȱ�ʾ)ԭ�Ӱ뾶����Դ�С��������ۻ�����۵ı仯����ͼ��ʾ��
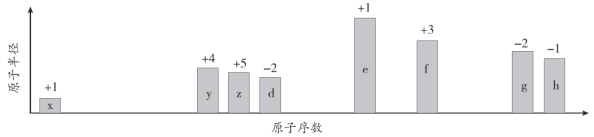
�����жϳ���Ԫ�ػش����⣺
��1��f��Ԫ�����ڱ���λ����__________________��
��2���Ƚ�d��e�������ӵİ뾶��С(�û�ѧʽ��ʾ����ͬ)______��_______���Ƚ�g��h������������Ӧ��ˮ���������ǿ���ǣ�_______��____��
��3������x2d2�ĵ���ʽ��____________________��
��4����֪1mol e�ĵ���������d2��ȼ�գ��ָ������£��ų�255.5kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��____________________________________________��
��5��д���ö��Ե缫���eh��Һ�Ļ�ѧ����ʽ��________________________________��
���𰸡��������ڢ� A�� r(O2-) r(Na+) HClO4 H2SO4 ![]() 2Na(s)+O2(g)=Na2O2(s) ��H= -511kJ��mol-1
2Na(s)+O2(g)=Na2O2(s) ��H= -511kJ��mol-1 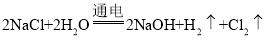
��������
ͬ���ڴ�������ԭ�Ӱ뾶���μ�С��ͬ������ϵ���ԭ�Ӱ뾶������������Ԫ�ص�������۵���������������۵ľ���ֵ=8���������������ݴ˷�������xΪH��yΪC��zΪN��dΪO��eΪNa��fΪAl��gΪS��hΪCl���ݴ˷�����
����������ۻ�����ۣ��Լ�ԭ��������ԭ�Ӱ뾶���Ƴ�xΪH��yΪC��zΪN��dΪO��eΪNa��fΪAl��gΪS��hΪCl��
��1��fΪAl��λ�ڵ������ڵڢ� A�壻
��2��d��e��������O2-��Na+�����Ǻ�������Ų���ͬ��ԭ������Խ�뾶ԽС����r(O2-)>r(Na+)���ǽ�����Խǿ��������������Ӧˮ���������Խǿ��gΪS��hΪCl��Cl�ķǽ�����ǿ��S����HClO4������ǿ��H2SO4��
��3��x2d2�Ļ�ѧʽΪH2O2����ṹʽΪH-O-O-H������ʽΪ![]() ��
��
��4��Na��������ȼ������Na2O2����1mol Na��������ȼ�գ�����Na2O2���Ȼ�ѧ��Ӧ����ʽΪNa(s)+![]() O2(g)=
O2(g)= ![]() Na2O2(s) ��H= -255.5kJ��mol-1����2Na(s)+O2(g)=Na2O2(s) ��H= -511kJ��mol-1��
Na2O2(s) ��H= -255.5kJ��mol-1����2Na(s)+O2(g)=Na2O2(s) ��H= -511kJ��mol-1��
��5�����Բ��ϵ�ⱥ��ʳ��ˮ���䷴Ӧ����ʽΪ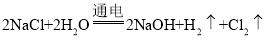 ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��25��ʱ���������ʵĵ���ƽ�ⳣ�������ʾ��
��ѧʽ | CH3COOH | H2CO3 | HC1O |
����ƽ�ⳣ�� | 1.7��10-5 | K1=4.3��10-7 K2=5.6��10-11 | 3.0��10-8 |
��ش��������⣺
��1��CH3COOH��H2CO3��HC1O��������ǿ������˳��Ϊ______________________��
��2��������0.1 mol��L-1��CH3COOH��Һ�������{�¶ȣ�����4�ֱ���ʽ�������������______��
A��c(H+) B��c(H+)/c(CH3COOH) C�� c(H+)��c(OH-) D��c(CH3COO��)��c(H+)/c(CH3COOH)
��3��ȡ0.10mol CH3COOH �������ᣩ��������ʵ�飬����䵼����������ˮ���仯��ͼ��ʾ���Ƚ�a��b���������ʣ��>����<����=������

n(H+)��a_____b��c(CH3COO-)��a_____b����ȫ�к�ʱ����NaOH�����ʵ�����a_____b��
��4��H+Ũ����ͬ�������������ҺA(���ᣩ��B(CH3COOH)�քe��п�۷�Ӧ����������һ����Һ�д���п���ų�������������ͬ��������˵����ȷ����__________����д��ţ�
�ٷ�Ӧ����Ҫ��ʱ��B>A �ڿ�ʼ��Ӧʱ������A>B
�۲μӷ�Ӧ��п�����ʵ���A=B ��A����пʣ��