��Ŀ����
��֪�����Ȼ�ѧ����ʽ��
��H2O��l���TH2��g��+
O2��g����H=+285.8kJ/mol
��H2��g��+
O2��g���TH2O��g����H=-241.8kJ/mol
��NaOH��aq��+HCl��aq���TNaCl��aq��+H2O��l����H=-57.3kJ/mol
��C��s��+
O2��g���TCO��g����H=-110.5kJ/mol
��C��s��+O2��g���TCO2��g����H=-393.5kJ/mol
�ش��������⣺
��1��������Ӧ���������ȷ�Ӧ���� ��
��2��C��ȼ����Ϊ ��
��3��ȼ��10g H2����Һ̬ˮ���ų�������Ϊ ��
��4��д����ˮú�����Ȼ�ѧ����ʽ�� ��
��H2O��l���TH2��g��+
| 1 |
| 2 |
��H2��g��+
| 1 |
| 2 |
��NaOH��aq��+HCl��aq���TNaCl��aq��+H2O��l����H=-57.3kJ/mol
��C��s��+
| 1 |
| 2 |
��C��s��+O2��g���TCO2��g����H=-393.5kJ/mol
�ش��������⣺
��1��������Ӧ���������ȷ�Ӧ����
��2��C��ȼ����Ϊ
��3��ȼ��10g H2����Һ̬ˮ���ų�������Ϊ
��4��д����ˮú�����Ȼ�ѧ����ʽ��
���㣺��Ӧ�Ⱥ��ʱ�,�Ȼ�ѧ����ʽ
ר�⣺��ѧ��Ӧ�е������仯
��������1����H��0�ķ�Ӧ�����ȷ�Ӧ��
��2��1molC��ȫȼ�����ɶ�����̼ʱ�ų���������C��ȼ���ȣ�
��3���ٵ��淴Ӧ��������ȼ������Һ̬ˮʱ����ЧӦ���ݴ˽��
��4�����ø�˹���ɽ�𣬽���-�ڿɵã�
��2��1molC��ȫȼ�����ɶ�����̼ʱ�ų���������C��ȼ���ȣ�
��3���ٵ��淴Ӧ��������ȼ������Һ̬ˮʱ����ЧӦ���ݴ˽��
��4�����ø�˹���ɽ�𣬽���-�ڿɵã�
���
�⣺��1����H��0�ķ�Ӧ�����ȷ�Ӧ���ʴ�Ϊ���٣�
��2��C��ȼ������1molC���ɶ�����̼ʱ�ų����������ʴ�Ϊ��393.5 kJ/mol��
��3����H2O��l���TH2��g��+
O2��g����H=+285.8kJ/mol��֪��2g����ȼ������Һ̬ˮʱ����285.8KJ����10g����ȼ�շ���5��285.8KJ=1429 kJ��
�ʴ�Ϊ��1429 kJ��
��4����֪��H2��g��+
O2��g���TH2O��g����H=-241.8kJ/mol
��C��s��+
O2��g���TCO��g����H=-110.5kJ/mol
�ݸ�˹���ɣ���-�ڵã�C��s��+H2O��g���TH2��g��+CO��g����=+131.3 kJ/mol��
�ʴ�Ϊ��C��s��+H2O��g���TH2��g��+CO��g����=+131.3 kJ/mol��
��2��C��ȼ������1molC���ɶ�����̼ʱ�ų����������ʴ�Ϊ��393.5 kJ/mol��
��3����H2O��l���TH2��g��+
| 1 |
| 2 |
�ʴ�Ϊ��1429 kJ��
��4����֪��H2��g��+
| 1 |
| 2 |
��C��s��+
| 1 |
| 2 |
�ݸ�˹���ɣ���-�ڵã�C��s��+H2O��g���TH2��g��+CO��g����=+131.3 kJ/mol��
�ʴ�Ϊ��C��s��+H2O��g���TH2��g��+CO��g����=+131.3 kJ/mol��
������������Ҫ���������ȷ�Ӧ�ı�ʾ��ȼ���ȡ���Ӧ�ȵļ��㡢��˹���ɵ�Ӧ�ã���Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
���������в���ȷ���ǣ�������
| A��Ŀǰ���ҹ����л�����Ⱦ�еĴ�����Ⱦ����Ҫ��SO2��NO2��CO���̳� |
| B��������ʳƷ���Ӽ���ʳ������彡�����к�������ʳ�� |
| C������̫���ܵ������Դ���滯ʯȼ�ϣ������ڽ�Լ��Դ���������� |
| D��Ϊ��ֹ����е��ؽ�������Ⱦ������ˮ�壬Ӧ���������ϵ�ص��ۺ����ü��� |
����������Ʒ�ڸ�ʴ�����к����ױ���ʴ����ʹ��������Ϊ���ͣ����ֻ�жԸ�ʴ�����еĽ���������Ʒ��ȡ������ʩ�����ܱ�֤���������豸�İ�ȫ�Ժ�ũҵ������˳�����У�����Խ���������Ʒ�ķ�����ʩ�д�����ǣ�������
| A�������������ˮˢȥ���������ۣ��ó�ʪ������ |
| B��ͨ������գ���������Ʒ���������Ĥ |
| C�����������������Ʒ�����������Ҫ����Ϳˢ������ |
| D����Cr��Ni�Ƚ������뵽��ͨ�����Ƴɲ���� |
ij����ʵ��С����Ƶ�����ʵ�鲻�������ǣ�������
A��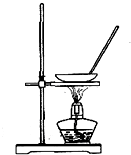 ����NH4Cl������Һ�Ʊ�NH4Cl���� |
B�� ʵ�����Ʊ��������� |
C��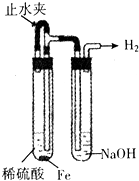 �Ʊ����۲����������� |
D��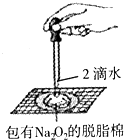 ֤������������ˮ��Ӧ���� |
���������У�������NaOH��Һ���������ᷴӦ���������ǣ�������
| A��Al2O3 |
| B��Al |
| C��Al��OH��3 |
| D��NaHCO3 |
�йػ�ѧ���ͻ��������������ȷ���ǣ�������
| A�������к���H+��Cl-������HCl�����ӻ����� |
| B��Na2O2�к������Ӽ��ͷǼ��Թ��ۼ� |
| C����ȫ�ɷǽ���Ԫ����ɵĻ�������������ӻ����� |
| D��˫ԭ�ӻ��ԭ�ӵĵ��ʷ����о����ڻ�ѧ�� |