��Ŀ����
��˾ƥ�ֵ���Ч�ɷ�������ˮ���ᣨ ����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳ�����ˮ���ᣬ�Ʊ�����Ҫ��ӦΪ��
����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳ�����ˮ���ᣬ�Ʊ�����Ҫ��ӦΪ��
�����������£�
��֪��ˮ���������ˮ���������ˮ����������������ˮ����������ˮ�ֽ����ɴ��ᡣ
�ش��������⣺
��1���ϳɹ���������ʵļ��ȷ����� ��
��2���Ʊ������У�ˮ������γɾۺ���ĸ����д���þۺ���Ľṹ��ʽ ��
��3���ֲ�Ʒ�ᴿ��
�� ��������������NaHCO3�ܽ�ֲ�Ʒ��Ŀ���� ���жϸù��̽����ķ����� ��
�� ��Һ��������Ũ�����У������������� ��
�� �������ղ�Ʒ���Ƿ���ˮ����Ļ�ѧ������ ��
��4����˾ƥ��ҩƬ������ˮ���Ậ���IJⶨ���裨�ٶ�ֻ������ˮ������ϣ����ϲ����뷴Ӧ����
��.��ȡ��˾ƥ����Ʒm g����.����Ʒ���飬����V1 mL a mol��L-1NaOH�������������ȣ���ȥ���ϵȲ������������Һ������ƿ����.����ƿ�еμӼ��μ��ȣ���Ũ��Ϊb mol��L-1�ı����ᵽ�ζ�ʣ���NaOH��������������ΪV2mL��
�� д������ˮ���������NaOH��Һ���ȷ�����Ӧ�Ļ�ѧ����ʽ
��
�� ��˾ƥ��ҩƬ������ˮ�������������ı���ʽΪ ��
26����12�֣�
��1��ˮԡ���ȣ�1�֣�����2�� 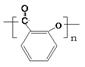 ��2�֣�
��2�֣�
��3����ʹ����ˮ����ת��Ϊ������ˮ������ˮ�����ƣ�������ۺ�����루1�֣���û��CO2������1�֣������л��Dz�����1�֣�����ȡ�����ᾧ���Թ��У�������ˮ�ܽ⣬�μ�FeCl3��Һ����������ɫ��ˮ���ᡣ��2�֣� 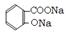 ��4����
��4����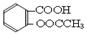 +3NaOH
+3NaOH CH3COONa+2H2O+ ��2�֣�
CH3COONa+2H2O+ ��2�֣�
��0.180 (aV1-bV2) /3m��2�֣�
���������������1���¶Ȳ�����100�棬�ϳɹ���������ʵļ��ȷ�����ˮԡ���ȡ���2���Ʊ������У�ˮ����ᷢ���������۷�Ӧ������ ����3���ֲ�Ʒ�ᴿ���� ��������������NaHCO3�ܽ�ֲ�Ʒ��Ŀ����ʹ����ˮ����ת��Ϊ������ˮ������ˮ�����ƣ�������ۺ�����롣�жϸù��̽����ķ�����û��CO2�������� ��Һ��������Ũ�����У��������������л��Dz������� �������ղ�Ʒ���Ƿ���ˮ����Ļ�ѧ������ȡ�����ᾧ���Թ��У�������ˮ�ܽ⣬�μ�FeCl3��Һ����������ɫ��ˮ���ᡣ��4���� ����ˮ���������NaOH��Һ���ȷ�����Ӧ�Ļ�ѧ����ʽ
����3���ֲ�Ʒ�ᴿ���� ��������������NaHCO3�ܽ�ֲ�Ʒ��Ŀ����ʹ����ˮ����ת��Ϊ������ˮ������ˮ�����ƣ�������ۺ�����롣�жϸù��̽����ķ�����û��CO2�������� ��Һ��������Ũ�����У��������������л��Dz������� �������ղ�Ʒ���Ƿ���ˮ����Ļ�ѧ������ȡ�����ᾧ���Թ��У�������ˮ�ܽ⣬�μ�FeCl3��Һ����������ɫ��ˮ���ᡣ��4���� ����ˮ���������NaOH��Һ���ȷ�����Ӧ�Ļ�ѧ����ʽ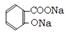
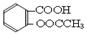 +3NaOH
+3NaOH CH3COONa+2H2O+ ���� ���ݷ�Ӧ����ʽ���ù�ϵʽ��
CH3COONa+2H2O+ ���� ���ݷ�Ӧ����ʽ���ù�ϵʽ�� �D3NaOH�����ݵζ����������ǵõ�����ˮ����������
�D3NaOH�����ݵζ����������ǵõ�����ˮ����������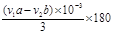 g����˾ƥ��ҩƬ������ˮ�������������ı���ʽΪ
g����˾ƥ��ҩƬ������ˮ�������������ı���ʽΪ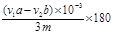 ��
��
���㣺�����л���ṹ��������֮����ת��������ؼ��㡣

(12��)ijʵ��С��������װ�ý����Ҵ���������ʵ�顣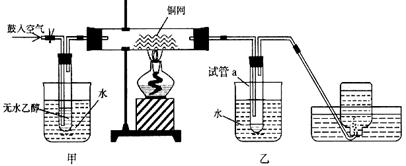
��1��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ����ʽ_________________��
��2����������ˮԡ���ò���ͬ���ҵ�������___________________������ƿ���ռ������������Ҫ�ɷ���_____________________��
��3�����Թ�a���ռ�����Һ������ɫʯ����ֽ����ֽ�Ժ�ɫ��˵��Һ���л���____________��Ҫ��ȥ�����ʣ������ڻ��Һ�м���_________����д��ĸ����
| A���Ȼ�����Һ | B���� | C��̼��������Һ | D�����Ȼ�̼ |
��4��ʵ��һ��ʱ����Թ�a�е�Һ��ȡ��������������������Һ���ٷ�����ձ��У��ܿ�������Ե�����д���÷�Ӧ�Ļ�ѧ����ʽ�� ����Ӧ����Ϊ
����ȩ��һ�ֻ���ԭ�ϡ�ijʵ��С����������װ�úϳ�����ȩ��
�����ķ�Ӧ���£�
CH3CH2CH2CH2OH CH3CH2CH2CHO
CH3CH2CH2CHO
��Ӧ��Ͳ������������б����£�
| | �е�/��c | �ܶ�/(g��cm-3) | ˮ���ܽ��� |
| ������ | 11.72 | 0.8109 | �� |
| ����ȩ | 75.7 | 0.8017 | �� |
ʵ�鲽�����£�
��6.0gNa2Cr2O7����100mL�ձ��У���30mLˮ�ܽ⣬�ٻ�������5mLŨ���ᣬ��������ҺС��ת����B�С���A�м���4.0g�������ͼ�����ʯ�����ȡ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90��95�棬��E���ռ�90�����µ���֡�
������ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75��77����֣�����2.0g��
�ش��������⣺
��1��ʵ���У��ܷ�Na2Cr2O7��Һ�ӵ�Ũ�����У���˵������ ��
��2�������ʯ�������� �������Ⱥ���δ�ӷ�ʯ��Ӧ��ȡ����ȷ������ ��
��3������װ��ͼ�У�B������������ ��D������������ ��
��4����Һ©��ʹ��ǰ������еIJ����� ������ȷ�𰸱�ţ���
a.��ʪ b.���� c.��© d.�궨
��5��������ȩ�ֲ�Ʒ���ڷ�Һ©���з�ˮʱ��ˮ�� �㣨��ϡ����¡���
��6����Ӧ�¶�Ӧ������90��95�棬��ԭ���� ��
��7����ʵ���У�����ȩ�IJ���Ϊ %��
���й�����ϩ�;���ϩ����������ȷ����
| A����ϩ�����������壬Ϊ���������ϩ�������ǹ��壬Ϊ����� |
| B����ϩ�Ļ�ѧ���ʱȾ���ϩ���� |
| C��ȡ����������ϩ�;���ϩ��ȫȼ�պ����ɵ�CO2��H2O�������ֱ���� |
| D��ȡ�����ʵ�������ϩ�;���ϩ��ȫȼ�պ����ɵ�CO2��H2O�����ʵ����ֱ���� |
��ѧ��������������ء�����˵����ȷ���ǣ� ����
| A��Si��������Ϣ���ٹ�·�ĹǼܡ��� ���ά����Ҫ���� |
| B���������ѷ��������������ձ�������CO2��NO2�Ϳ�������������������Ҫ��Ⱦ�� |
| C��ú̿��������Һ������ȹ��̣��ɻ�������Դ����Ҫ�Ļ���ԭ�� |
| D��SO2��������Ư��ֽ����ë��˿����ñ�衢����ʳƷ�� |
�л����ұ� ��һ��ȡ�����ͬ���칹���У� ��
��һ��ȡ�����ͬ���칹���У� ��
| A��5�� | B��6�� | C��7�� | D��8�� |
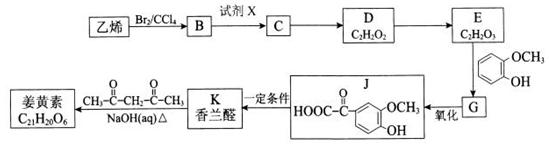




 ���е���131.4�棬�۵���9.79�棬������ˮ�������ڴ�����ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾװ�����Ʊ�1��2-�������顣�����Թ�c��װ��Һ�壨���渲������ˮ����
���е���131.4�棬�۵���9.79�棬������ˮ�������ڴ�����ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾװ�����Ʊ�1��2-�������顣�����Թ�c��װ��Һ�壨���渲������ˮ����