��Ŀ����
��ҵ���ù�������������ȡ�������죨Fe2O3������Ӧԭ���ǣ�?![]()
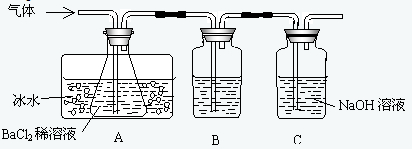
ij�о���ѧϰС������ͼ��ʾװ�÷ֱ����÷�Ӧ����������̬���ʣ�Ȼ������װ��Aƿ�ڵ����������ⶨ�ѷֽ��FeSO4������������֪��SO2�е�Ϊ-10.02 �棩?
��ش�������⣺?
��1����ʵ���У�Aƿ��ʢBaCl2��Һ�������յ�������________��������Ӧ�����ӷ���ʽΪ________________________��
��2��Bƿ��ʢ���Լ���������________________��Cƿ?��ʢ�Լ���������________________��
��3��AƿҪ����ˮ��ȴ��ԭ����________________________��?
��4��ijѧ��������װ��B��Ӧǰ���������ȷ���ѷֽ��FeSO4����������Ϊ��ͬѧ�����Ƿ����________�����������������������������________________________��?
��5����������װ�úͷ�Ӧ��ʵ������Ʋⶨ�ѷֽ��FeSO4�����IJ����ͷ�����________��
��1��SO3��SO3+H2O+Ba 2+�T�TBaSO4��+2H +??
��2���������ɵ�SO2����δ��ȫ��Ӧ��SO2����ֹ��Ⱦ����?
��3����ΪSO3�ܽ���ȣ���ֹ�γ�������ʹSO3������ȫ?
��4�������� Ʒ����Һ���ܽ�SO2��ȫ����??
��5����װ��A�еij������ˡ�ϴ�ӡ�������������ݳ��������BaSO4��������������ѷֽ���������������?
��������1����ΪSO2��BaCl2����Ӧ��

 ������ÿ�ʱ�Ż���ҵϵ�д�
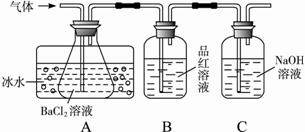
������ÿ�ʱ�Ż���ҵϵ�д���ҵ���ù�������������ȡ��������(Fe2O3)��Ӧԭ���ǣ�2FeSO4Fe2O3��SO2����SO3����ijѧ��������÷�Ӧ����������̬���ʣ����ν�����ͨ��ʢ��(��)BaCl2��Һ��(��)X��Һ��(��)NaOH��Һ������װ�á������жԸ÷�������������ȷ���ǣ� ��
| A�������л�����BaSO3��BaSO4���ֳ��� |
| B���ɽ������е�BaCl2��Һ��ΪBa(NO3)2��Һ |
| C��������ʢX��ΪƷ����Һ |
| D�������������������SO2���� |