��Ŀ����
18����NAΪ�����ӵ�������ֵ������˵����ȷ���ǣ�������| A�� | 0.1molZn�뺬0.1molHCl�������ַ�Ӧ��ת�Ƶĵ�����ĿΪ0.2NA | |
| B�� | 1molNa������O2��Ӧ������Na2O��Na2O2�Ļ���ת�Ƶĵ�����ΪNA | |
| C�� | 1molNa2O2������CO2��ַ�Ӧת�Ƶĵ�����Ϊ2NA | |
| D�� | ��FeI2��Һ��ͨ������Cl2������1molFe2+������ʱ����ת�Ƶĵ��ӵ���ĿΪNA |
���� A��0.1molZn�뺬0.1molHCl�����ᷴӦʱ��п������
B�����ݷ�Ӧ����Ԫ��Ϊ+1����������
C������������ˮ�ķ�ӦΪ�绯��Ӧ��
D�����ݵ����Ӻ��������Ӷ��ܹ��������������������������������������ӷ�����
��� �⣺A��0.1molZn�뺬0.1molHCl�����ᷴӦʱ��п������0.1mol������ȫ��Ӧ��ת��0.1mol���Ӽ�0.1NA������A����
B�����ڷ�Ӧ����Ԫ��Ϊ+1�ۣ���1mol�Ʒ�Ӧת��1mol���Ӽ�NA����������أ���B��ȷ��
C��1mol Na2O2������CO2��ַ�Ӧת�Ƶĵ�����ΪNA����C����
D��FeI2��Һ�У������ӵĻ�ԭ�Դ����������ӵģ�ͨ����������������1molFe2+������ʱ����Һ�е������Ѿ���ȫ�����������ڲ�֪���⻯���������ʵ�����������ת�Ƶĵ���������D����
��ѡB��
���� ���⿼���˰���٤���������йؼ��㣬�������չ�ʽ��ʹ�ú����ʵĽṹ�ǽ���ؼ����ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
9����NA��ʾ����ӵ�����������˵����ȷ���ǣ�������
| A�� | ��״���£�2.24Lˮ�����ķ�����ĿΪ0.1 NA | |
| B�� | 9gˮ�к��еĵ�����ĿΪ0.5NA | |
| C�� | 0.3 mol/L��MgCl2��Һ�к�Mg2+��ĿΪ0.3 NA | |
| D�� | ����ѹ�£�28gN2��CO��������������е�ԭ����ĿΪ2NA |
6������ɫ��Һ�����и��������ܴ���������ǣ�������
| A�� | Ag+��K+��NO${\;}_{3}^{-}$��Cl- | B�� | Mg2+��Na+��Cl-��SO${\;}_{4}^{2-}$ | ||
| C�� | Ca2+��Mg2+��Fe2+��Cl- | D�� | H+��Na+��CO${\;}_{3}^{2-}$��SO${\;}_{4}^{2-}$ |
7�����й��ڽ������������ȷ���ǣ�������
| A�� | ����������������ɢϵ�ı��������Ƿ�ɢ�ʵ���ֱ����1nm��100nm֮�� | |
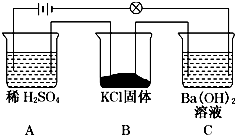
| B�� | ��ƽ�й�����CuSO4��Һ��Fe��OH��3���壬���Լ������� | |
| C�� | ��FeCl3������Һ���뵽NaOH��Һ�У�����ȡFe��OH��3���� | |
| D�� | �����еij��أ��������붡���ЧӦ�й� |
8������ͼʽ��ʾ��ȷ���ǣ�������
| A�� | ������̼ | B�� | ������Ľṹʽ H-Cl-O | ||
| C�� | �����ӵĽṹʾ��ͼ | D�� | ��̬̼ԭ�ӵļ۵����Ų�ͼΪ |

 ��1��A��B��C��D��F�������ʵ���ɫ��Ӧ��Ϊ��ɫ��A��B��C��D�����ᷴӦ������E������B������һ�ֿ�ȼ�����壮��C��D������һ����ɫ��ζ����H����������ʹ����ʯ��ˮ����ǣ�D��A�ɷ�Ӧ����C��F��HҲ�ɷ�Ӧ����C����һ����ɫ��ζ���壮��ش��������⣺
��1��A��B��C��D��F�������ʵ���ɫ��Ӧ��Ϊ��ɫ��A��B��C��D�����ᷴӦ������E������B������һ�ֿ�ȼ�����壮��C��D������һ����ɫ��ζ����H����������ʹ����ʯ��ˮ����ǣ�D��A�ɷ�Ӧ����C��F��HҲ�ɷ�Ӧ����C����һ����ɫ��ζ���壮��ش��������⣺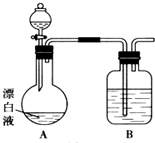 ij��ѧ��ȤС��ͬѧ����һ����ij��ֽ���۳���Ư��Һ��NaCl��NaClO�Ļ��Һ������ʢ�ű���KAl��SO4��2��Һ�ij��У�����ж��¼�����С��ͬѧΪ̽���ж�ԭ�����������ʵ�飮
ij��ѧ��ȤС��ͬѧ����һ����ij��ֽ���۳���Ư��Һ��NaCl��NaClO�Ļ��Һ������ʢ�ű���KAl��SO4��2��Һ�ij��У�����ж��¼�����С��ͬѧΪ̽���ж�ԭ�����������ʵ�飮 ���еĹ��������������Ȼ���д���ƣ����ð�˾ƥ�ֺ����������к��Ƶÿ�����˾ƥ�֣��÷�Ӧ�Ļ�ѧ����ʽΪC6H5OOCCH3COOH+H2O��CH3COOH+C6H5OHCOOH��
���еĹ��������������Ȼ���д���ƣ����ð�˾ƥ�ֺ����������к��Ƶÿ�����˾ƥ�֣��÷�Ӧ�Ļ�ѧ����ʽΪC6H5OOCCH3COOH+H2O��CH3COOH+C6H5OHCOOH��