��Ŀ����
����Ŀ��ij����С�����þ��CO2�ķ�Ӧ���ʵ��̽��þ��NO2�ķ�Ӧ��
��ͬѧ�Ʋ������MgO��N2��
��ͬѧ�Ʋ�������MgO��N2�⣬�����л����ܺ���Y��
��С��ͬѧ���������װ��̽��þ��NO2��Ӧ�Ĺ��������ⶨ����ɡ�

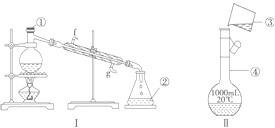
��1��ʵ�鿪ʼʱ���ȹر�ֹˮ�к���ɼУ��ٴ�Һ©����������Ӳ�ʲ����ܳ�������ɫ�����ֹˮ�У��رյ��ɼУ�����ȼ�ƾ��ơ���������Ŀ����___________________________________________________________
��2��װ��B�е��Լ�����ѡ��________
A��Ũ���� B����ˮ�Ȼ��� C������������ D����ʯ��
��3��װ��C��ʢװ����������Һ�������ǣ�___________________________________
��4��Ϊ��֤�������������ȷ�ԣ���ȡ��Ͳ�̶�ʱӦע��������Ǣ�����ָ��������ٶ�������_______________________________����______________________________��
��5��ʵ���������ͬѧ�ǽ��������ȡ����ˮ��Ӧ�������д̼�����ζ�������������������ʹʪ���ʯ����ֽ������˵������ͬѧ�Ʋ���ȷ����д��Y��ˮ��Ӧ�Ļ�ѧ����ʽ__________________________________
��6������ʼ����þ������Ϊ3.6 g����������NO2�г�ַ�Ӧ�� ���ռ���N2���Ϊ448mL (��״��)���������MgO��������_________
���𰸡� �ų�װ���п���������������� BC ����ʣ��NO2 ������Ͳ��Һ����D��ˮƽ ������Һ�尼Һ������ Mg3N2+H2O=3Mg(OH)2��+2NH3�� 4.8g
��������(1)ʵ�鿪ʼʱ���ȹر�ֹˮ�к���ɼУ��ٴ�Һ©����������Ӳ�ʲ����ܳ�������ɫ�����ֹˮ�У��رյ��ɼУ�����ȼ�ƾ��ơ������������ų�װ���п���������������ţ��ʴ�Ϊ���ų�װ���п���������������ţ�
(2)װ��A��Ũ������ͭ��Ӧ���ɶ������������ɵĶ��������л���ˮ������װ��B�е��Լ���Ҫ��ȥ���е�ˮ����������ѡ����ˮ�Ȼ��ƺ��������������Թ�����������ѡBC��
(3)���������ܹ���Ⱦ������������ˮ��Ӧ����һ��������Ӱ�쵪��������IJⶨ��װ��C��ʢװ����������Һ��������Ϊ��Ӧ��NO2���ʴ�Ϊ������ʣ���NO2��
(4)Ϊ��֤�������������ȷ�ԣ���ȡ��Ͳ�̶�ʱӦע��������ǣ�������ָ��������ٶ������ڵ�����Ͳ��Һ����D��ˮƽ����������Һ�尼Һ�����У��ʴ�Ϊ��������Ͳ��Һ����D��ˮƽ��������Һ�尼Һ�����У�
(5)ʵ�������ͬѧ�ǽ��������ȡ����ˮ��Ӧ�������д̼�����ζ�������������������ʹʪ���ʯ����ֽ������˵�����ɵ�����Ϊ���������������к��е�Ԫ�أ����ݲ��뷴Ӧ������Ϊþ�Ͷ��������������жϹ������ΪMg3N2��Mg3N2��ˮ��Ӧ�Ļ�ѧ����ʽΪMg3N2+H2O=3Mg(OH)2��+2NH3�����ʴ�Ϊ��Mg3N2+H2O=3Mg(OH)2��+2NH3����
(6)þ�����ʵ���=![]() =0.15mol����Ӧ��������þ��þ��ʧȥ����0.3mol����״���£�N2���Ϊ448mL�����ʵ���=
=0.15mol����Ӧ��������þ��þ��ʧȥ����0.3mol����״���£�N2���Ϊ448mL�����ʵ���=![]() =0.02mol���õ��˵���0.02mol��8=0.16mol�����ݵ�ʧ�����غ㣬����Mg3N2
=0.02mol���õ��˵���0.02mol��8=0.16mol�����ݵ�ʧ�����غ㣬����Mg3N2![]() mol=0.01mol������þԭ���غ㣬���ɵ�����þΪ
mol=0.01mol������þԭ���غ㣬���ɵ�����þΪ![]() mol=0.12mol������Ϊ0.12mol��40g/mol=4.8g���ʴ�Ϊ��4.8g��
mol=0.12mol������Ϊ0.12mol��40g/mol=4.8g���ʴ�Ϊ��4.8g��

 �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�