��Ŀ����
Ϊ��̽��SO2��Na2O2�ķ�Ӧ�Ƿ�������CO2��Na2O2�ķ�Ӧ����ͬѧ�������ͼ��ʾ��ʵ��װ�ã��ش��������⣺
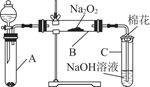
��1���ƿ������������ǵ�ľ������C�Թܿڣ�δ��ľ����ȼ����ͬѧ�����ΪSO2��Na2O2�ķ�Ӧ��ͬ��CO2���밴��ͬѧ�Ĺ۵�д����Ӧ�Ļ�ѧ����ʽ ��
��2����ͬѧ��Ϊ���۷�Ӧԭ����Σ����ն���O2��������ͬѧ�������� ��������ͬѧ�Ĺ۵㣬��װ�������ĸĽ���
��
��3������Na2O2��ȫ��Ӧ����Ӧ��Bװ���й�������������ǣ���Na2SO3����Na2SO4����Na2SO3��Na2SO4��
�����ʵ�鷽�����飬д��ʵ�鲽���Լ�Ԥ������ͽ��ۣ�����±���
��ѡ�Լ���2 mol��L��1 HCl��Һ��1 mol��L��1 HNO3��Һ��1 mol��L��1 BaCl��Һ��1 mol��L��1 Ba��NO3��2��Һ��0.01 mol��L��1 KMnO4������Һ��
��4�����������������ƺ����IJⶨ��
��ȡa g���������Ƴ�100 mL��Һ��ȡ10.00 mL����Һ����ƿ�У����뼸�ε�����Һ��ָʾ������0.010 0 mol��L��1��ˮ���еζ����ζ��յ�����Ϊ ����¼���ݣ��ظ��ζ�2�Σ�ƽ�����ĵ�ˮ20.00 mL��
�ڼ��㣺���������������Ƶ���������Ϊ ��

��1���ƿ������������ǵ�ľ������C�Թܿڣ�δ��ľ����ȼ����ͬѧ�����ΪSO2��Na2O2�ķ�Ӧ��ͬ��CO2���밴��ͬѧ�Ĺ۵�д����Ӧ�Ļ�ѧ����ʽ ��
��2����ͬѧ��Ϊ���۷�Ӧԭ����Σ����ն���O2��������ͬѧ�������� ��������ͬѧ�Ĺ۵㣬��װ�������ĸĽ���
��
��3������Na2O2��ȫ��Ӧ����Ӧ��Bװ���й�������������ǣ���Na2SO3����Na2SO4����Na2SO3��Na2SO4��
�����ʵ�鷽�����飬д��ʵ�鲽���Լ�Ԥ������ͽ��ۣ�����±���
��ѡ�Լ���2 mol��L��1 HCl��Һ��1 mol��L��1 HNO3��Һ��1 mol��L��1 BaCl��Һ��1 mol��L��1 Ba��NO3��2��Һ��0.01 mol��L��1 KMnO4������Һ��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡB�е�����������Ʒ���Թ��У��μ���������ˮ���ܽ⣬Ȼ��ȡ��������Һ�ֱ����ڢ��Թ��� | ������ȫ�ܽ� |
| ����2�������Թ��м��� ���ٵμ� | �� |
| ��֤���������к�Na2SO4 | |
| ����3�������Թ��� | |
| | �� �� |
| ��֤������������Na2SO3���� | |
| | |
| ��˵����������û��Na2SO3�� | |
��4�����������������ƺ����IJⶨ��
��ȡa g���������Ƴ�100 mL��Һ��ȡ10.00 mL����Һ����ƿ�У����뼸�ε�����Һ��ָʾ������0.010 0 mol��L��1��ˮ���еζ����ζ��յ�����Ϊ ����¼���ݣ��ظ��ζ�2�Σ�ƽ�����ĵ�ˮ20.00 mL��
�ڼ��㣺���������������Ƶ���������Ϊ ��
��1��SO2��Na2O2=Na2SO4
��2��A�����ɵ�SO2�����к���ˮ����
��A��B֮������һ��װ��Ũ�����ϴ��ƿ�����������ʵĸ���װ�ã�
��3��
��4������Һ������ɫ���Ұ�����ڲ���ɫ
�� ��100%
��100%
��2��A�����ɵ�SO2�����к���ˮ����
��A��B֮������һ��װ��Ũ�����ϴ��ƿ�����������ʵĸ���װ�ã�
��3��
| ����2�������Թ��м���������1_mol��L��1�������ٵμ�1_mol��L��1_BaCl2��Һ | �а�ɫ������������֤���������к�Na2SO4 |
| ����3�������Թ�������2��3��0.01_mol��L��1_KMnO4������Һ���� | ��KMnO4��Һ�Ϻ�ɫ��ȥ����֤������������Na2SO3�� |
| ��KMnO4��Һ�Ϻ�ɫ����ȥ����˵����������û��Na2SO3 | |
��
 ��100%
��100%��1������2��ʵ���ԭ���ɽ��Ϊ������Ӧ����������ͨ������NaOH��Һ��ȥ����SO2�����岻��ʹ������ľ����ȼ˵����Ӧ��û��O2���ɣ�����ͬѧ�Ĺ۵��ǿ��ǵ�ˮ������Ӱ�죬����Ҫ���ʵ�齫�����������ô�����ľ�������Ƿ�ΪO2����3�����ݼ����е�������������ʵ���Ŀ�ľ��Ǽ������ù������Ƿ���SO42-��SO32-��SO42-�ļ��������BaCl2��ϡ���SO32-�ļ���ɸ����仹ԭ�ԣ�������Һ�Ƿ���ʹ���Ը��������Һ��ɫ�����С���4���ٵζ������з����ķ�ӦΪH2O��SO32-��I2=2I����SO42-��2H�����ʵζ��յ�ʱ������Ϊ��Һ������ɫ���Ұ�����ڲ���ɫ�����ɢ������ӷ���ʽ�ɵù�ϵʽNa2SO3��I2�������������������Ƶ���������Ϊ ��100%��
��100%�� ��100%��
��100%��
 ��100%��
��100%�� ��100%��
��100%��
��ϰ��ϵ�д�
�����Ŀ