��Ŀ����
��ʳ�γ���������K+��Ca2+��Mg2+��Fe3+��SO42-���������ӣ�ʵ�����ᴿNaCl���������£�
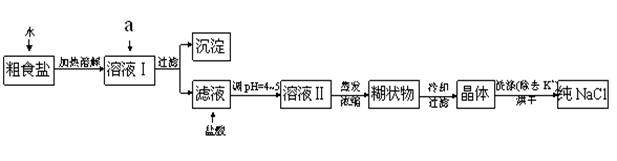
�ṩ���Լ�������Na2CO3��Һ�� ����K2CO3��Һ ��NaOH��Һ�� KOH��Һ�� BaCl2��Һ ��Ba��NO3��2��Һ
��1������ȥ��Һ�е�Ca2+��Mg2+��Fe3+��SO42-���ӣ�ѡ��a�������ĸ����Լ������μ�˳������Ϊ ���ѧʽ����
��2������Ũ����Һ��õ��ĺ�״��Ļ�ѧ�ɷ������ǣ��ѧʽ����
��3�����ᴿ����NaCl����������480 mL 0��4 mol��L-1NaCl��Һʱ�������������ձ���ҩ�ס�����������ƽ����ͷ�ι���� �����������ƣ�����NaCl g��
��4����ⱥ��ʳ��ˮ��װ����ͼ��ʾ��
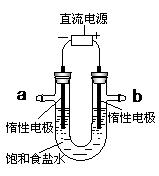
���ռ�����H2Ϊ2 L����ͬ���������ռ�����Cl2��� ���>������=����<����2 L����ԭ���ǣ� ��������������������װ�õ�b���ܷ�ס������һ��ʱ���U���п��Ի��һ������Һ��д����ô�����Һ��һ���ܷ�Ӧ����ʽ�� ��

�ṩ���Լ�������Na2CO3��Һ�� ����K2CO3��Һ ��NaOH��Һ�� KOH��Һ�� BaCl2��Һ ��Ba��NO3��2��Һ
��1������ȥ��Һ�е�Ca2+��Mg2+��Fe3+��SO42-���ӣ�ѡ��a�������ĸ����Լ������μ�˳������Ϊ ���ѧʽ����
��2������Ũ����Һ��õ��ĺ�״��Ļ�ѧ�ɷ������ǣ��ѧʽ����
��3�����ᴿ����NaCl����������480 mL 0��4 mol��L-1NaCl��Һʱ�������������ձ���ҩ�ס�����������ƽ����ͷ�ι���� �����������ƣ�����NaCl g��
��4����ⱥ��ʳ��ˮ��װ����ͼ��ʾ��

���ռ�����H2Ϊ2 L����ͬ���������ռ�����Cl2��� ���>������=����<����2 L����ԭ���ǣ� ��������������������װ�õ�b���ܷ�ס������һ��ʱ���U���п��Ի��һ������Һ��д����ô�����Һ��һ���ܷ�Ӧ����ʽ�� ��
����С��12�֣�
��1���μ�˳��Ϊ�� BaCl2 ��NaOH��Na2CO3 ����3�֣�����������Ҳ�ɣ�
��2����ѧ�ɷ��� NaCl ���ѧʽ������1�֣�
��3������ 500��������ƿ ��2�֣�δ�������ƿ��������11��7g��2�֣�
��4�� < 2 L��ԭ���ǣ� ������ˮ����һ�������ܽ� ������1�֣� �ܷ�Ӧ����ʽ��NaCl+H2O�� NaClO + H2������������⣩ ����2�֣�
��1���μ�˳��Ϊ�� BaCl2 ��NaOH��Na2CO3 ����3�֣�����������Ҳ�ɣ�
��2����ѧ�ɷ��� NaCl ���ѧʽ������1�֣�
��3������ 500��������ƿ ��2�֣�δ�������ƿ��������11��7g��2�֣�
��4�� < 2 L��ԭ���ǣ� ������ˮ����һ�������ܽ� ������1�֣� �ܷ�Ӧ����ʽ��NaCl+H2O�� NaClO + H2������������⣩ ����2�֣�
��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ��2�������ʵ�鷽��ȷ������Na2CO3��Һ�ѹ�����
��2�������ʵ�鷽��ȷ������Na2CO3��Һ�ѹ����� 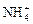 ��H����Cl����
��H����Cl����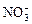 ��
��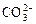 ��
��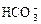 ��
��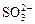 ��OH�������������У���ǿ������Һ�ﲻ���ܴ������ڵ���____________����ǿ������Һ�в����ܴ������ڵ�������____________���Ȳ�����ǿ������Һ��������ڣ�Ҳ������ǿ������Һ��������ڵ���______��������ǿ������Һ����ǿ������Һ������Դ������ڵ���____________
��OH�������������У���ǿ������Һ�ﲻ���ܴ������ڵ���____________����ǿ������Һ�в����ܴ������ڵ�������____________���Ȳ�����ǿ������Һ��������ڣ�Ҳ������ǿ������Һ��������ڵ���______��������ǿ������Һ����ǿ������Һ������Դ������ڵ���____________ ������ʵ������ļ��裬�������������Щ������һЩ�жϣ�
������ʵ������ļ��裬�������������Щ������һЩ�жϣ�
 �����ۣ�
�����ۣ�