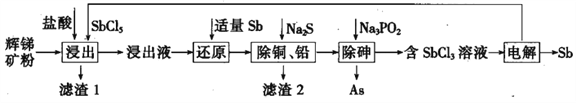
��Ŀ����
����Ŀ��ij�о���ѧϰС��ѧ������������ԭ��Ӧ���ɣ�̽��NO2��NO��Na2O2��Ӧ�������������貢�������ʵ�顣
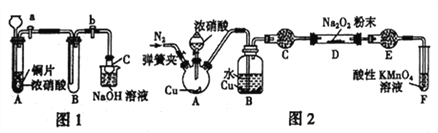
��.�������Ϸ���Na2O2��NO2���������������л�ԭ�ԣ�����������¼�����
����1��Na2O2����NO2������2��NO2 ����Na2O2��
��1����ͬѧ�����ͼ1װ�ý���ʵ����
���Թ�A �з�Ӧ�����ӷ���ʽ��____________________________��
�����Թ�B �г�������ɫ���壬�ر�����a��b��ȡ���Թ�B�������м�������Na2O2��ĩ���������ӣ��������Թ��ڷ�ĩ���۲쵽����ɫ����Ѹ����ʧ���ٽ������ǵ�ľ��Ѹ������Թ��ڣ�ľ����ȼ��
������C���з����������ã�����C������Ϊ___________________��
��������ͬѧ��Ϊ����2 ��ȷ��
��2����ͬѧ��Ϊ��ͬѧ��Ƶ�ʵ�����ȱ�ݣ�Ϊ�ﵽʵ��Ŀ�ģ���A��B֮��Ӧ����һ��װ�ã���װ�õ�������________________________����ͬѧ�øĽ����װ�ã��ظ��˼�ͬѧ��ʵ��������۲쵽����ɫ����Ѹ����ʧ�������ǵ�ľ��δ��ȼ���ó�����������1��ȷ����NO2��Na2O2��Ӧ�Ļ�ѧ����ʽ��_______________________��
��.���о���ѧϰС��ͬѧ����ΪNO ����O2������Ӧ��Ӧ�ø��ױ�Na2O2����������������2NO+Na2O2=2NaNO2��2NaNO2+2HCl=2NaCl+NO2��+NO��+H2O�� ���������£�NO ����MnO4-��Ӧ����NO3-��Mn2+��
��3����ͬѧ��ͼ2��ʾװ��(���ּг�װ����)̽��NO��Na2O2�ķ�Ӧ��
���ڷ�Ӧǰ�����ɼУ�ͨ��һ��ʱ��N2��Ŀ����_________________��
��B �й۲쵽����Ҫ������____________(����ĸ���)��
a.ͭƬ���ܽ⣬��Һ��Ϊ��ɫ b.�к���ɫ���ݲ��� C.����ɫ���ݲ�����C��E ����ʢװ���Լ�������________(����ĸ���)��
a.��ˮ����ͭ b.��ˮ�Ȼ��� c.��ʯ�� d.��ʯ��
��F �з�����Ӧ���������뻹ԭ�������ʵ���֮��Ϊ_____________��
����ַ�Ӧ����Dװ���в���ķ�������______________ ���������NaNO2��
���𰸡� Cu+4H++2NO3-=Cu2++2NO2��+2H2O ���θ���� ��ȥ����NO2�����л��е�ˮ���� Na2O2+2NO2=2NaNO3 ��װ���еĿ����ų� ac a 3��5 ȡDװ���в�������������ϡ���ᣬ��������ɫ����
����������1�����Թ�A��ͭ��Ũ���ᷴӦ�������ӷ���ʽ��Cu+4H++2NO3-=Cu2++2NO2��+2H2O���ڴ��Թ�B�г�������ɫ���壬�ر�����a��b��ȡ���Թ�B�������м�������Na2O2��ĩ���������ӣ��������Թ��ڷ�ĩ���۲쵽����ɫ����Ѹ����ʧ���ٽ������ǵ�ľ��Ѹ������Թ��ڣ�ľ����ȼ��������C���з����������ã�����CΪ�������
��2����ͬѧ��Ϊ��ͬѧ��Ƶ�ʵ�����ȱ�ݣ�Ϊ�ﵽʵ��Ŀ�ģ���A��B֮��Ӧ����һ��װ�ã���װ�õ������dz�ȥNO2�����л��е�ˮ��������ֹ�����ʵ�顣��ͬѧ�øĽ����װ�ã��ظ��˼�ͬѧ��ʵ��������۲쵽����ɫ����Ѹ����ʧ�������ǵ�ľ��δ��ȼ��ʹ�����ǵı�����ȼ��������ˮ������������Ʒ�Ӧ�õ����ɴ˵ó����ۣ�����1��ȷ�� NO2��Na2O2��Ӧ�Ļ�ѧ����ʽ��Na2O2+2NO2=2NaNO3��
��.���о���ѧϰС��ͬѧ����ΪNO����O2������Ӧ��Ӧ�ø��ױ�Na2O2�������������ϣ�2NO+Na2O2=2NaNO2��2NaNO2+2HCl=2NaCl+NO2��+NO��+H2O�����������£�NO����MnO4-��Ӧ����NO3-��Mn2+��
��3�����ڷ�Ӧǰ�����ɼУ�ͨ��һ��ʱ��N2��Ŀ���ǽ�װ���еĿ����ų�����ֹ��������ʵ������B�й۲쵽����Ҫ������ͭƬ���ܽ⣬��Һ��Ϊ��ɫ������ɫ���ݲ�����ѡac��.����ˮ����ͭͨ�����ڼ���ˮ������������ˮ������C��E����ʢװ���Լ�������a����F�з�������һ�����������Ը��������Һ�ķ�Ӧ�����е������������������ԭ����+2�������ӣ���Ԫ�صĻ��ϼ۽�����5����ԭ��NO�����������������Ԫ�صĻ��ϼ�������3�����ݵ�ʧ�����غ��֪���������뻹ԭ�������ʵ���֮��Ϊ3��5��
����������Ϣ��֪��2NaNO2+2HCl=2NaCl+NO2��+NO��+H2O����ˣ�����Dװ���в���ķ����ǣ�ȡDװ���в�������������ϡ���ᣬ��������ɫ���壬�������NaNO2��

 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�����Ŀ���о�̼�������仯�����ת�����ڻ����ĸ������ش����塣
(1) ������ԭ������NOx��ת������:
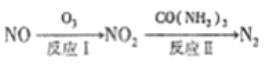
��֪: NO(g)+O3(g)=NO2(g)+O2(g) ��H=-200.9kJ/mol
2NO(g)+O2(g)=2NO(g) ��H=-116.2kJ/mol
��NO ��O3 ֻ����NO2 ���Ȼ�ѧ����ʽΪ_____________________��
(2) �������뽫CO�����з�Ӧ��ȥ: 2CO(g)=2C(s)+O2(g) ��H>0,��������÷�Ӧ�ܷ��Է�����?_____( ����������������)��������_____________________��
(3)����̿Ҳ�����ڴ�������β���е�NO����2L.�����ܱ������м���0.1000mo1NO��2.030mol �������̿������A��B�������壬�ڲ�ͬ�¶��²��ƽ����ϵ�и����ʵ����ʵ������±�:
�������̿/mol | NO/mol | A/mol | B/mol | |
200�� | 2.000 | 0.0400 | 0.0300 | 0.0300 |
335�� | 2.005 | 0.0500 | 0.0250 | 0.0250 |
������ϱ������ݣ�д��NO�����̿��Ӧ�Ļ�ѧ����ʽ______ �÷�Ӧ������ӦΪ_______(��������������������)��Ӧ��
��200��ʱ��ƽ���������������ٳ���0.1000mo1NO,�ٴ�ƽ���NO �����������____��(����������������С������������)��
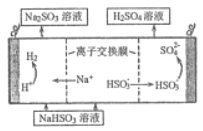
(4) ������������Һ���ն�������õ�������������Һ��Ȼ�������Һ���Ƶ����ᣬ���ԭ��ʾ��ͼ��ͼ��ʾ����д����ʼʱ�����ĵ缫��Ӧʽ______________��
(5)��������Ksp(BaCO3) =2.5��10-9,Ksp(BaSO4) =1.0��10-10������������ʵ�����³���ת��:
BaSO4(s)+CO32-(aq)=BaCO3(s)+SO42-(aq) ���÷�Ӧƽ�ⳣ��K�ı���ʽΪ:K=_______������1LNa2CO3 ��Һ��0.01molBaSO4 ȫ��ת��ΪBaCO3,��Na2CO3 ��Һ�����Ũ��Ӧ������_________��