��Ŀ����
�ӻ�������������ȡ�����ƵĹ������£�
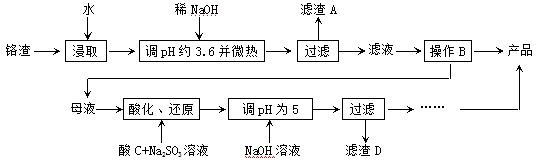
��֪���ٸ�������Na2SO4������Cr2O72-��Fe3+����Fe3+��Cr3+��ȫ������c ��1.0��10-5mol��L-1��ʱpH�ֱ�Ϊ3.6��5��
��1�����ȡ����ܼӿ췴Ӧ�����⣬ͬʱ������ ������AΪ ���ѧ
ʽ����
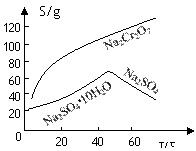
��2�������ܽ�ȣ�S�����¶ȣ�T�����ߣ�����B����ѷ���Ϊ ����
��ĸ��ţ�
A������Ũ�������ȹ��� B������Ũ�������½ᾧ������
��3���ữ��Cr2O72���ɱ�SO32-��ԭ��Cr3+�����ӷ���ʽΪ��
����CΪ ��Cr(OH)3���ܶȻ�����Ksp[Cr(OH)3]�� ��
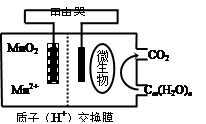
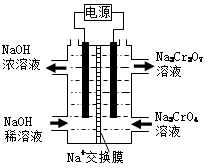
��4������2CrO42����2H+ Cr2O72����H2O���ͼʾװ�ã���Ϊ���Ե缫�����Na2CrO4��Һ��ȡNa2Cr2O7��ͼ���Ҳ�缫���ӵ�Դ�� ������缫��ӦʽΪ ��
Cr2O72����H2O���ͼʾװ�ã���Ϊ���Ե缫�����Na2CrO4��Һ��ȡNa2Cr2O7��ͼ���Ҳ�缫���ӵ�Դ�� ������缫��ӦʽΪ ��

��֪���ٸ�������Na2SO4������Cr2O72-��Fe3+����Fe3+��Cr3+��ȫ������c ��1.0��10-5mol��L-1��ʱpH�ֱ�Ϊ3.6��5��
��1�����ȡ����ܼӿ췴Ӧ�����⣬ͬʱ������ ������AΪ ���ѧ
ʽ����
��2�������ܽ�ȣ�S�����¶ȣ�T�����ߣ�����B����ѷ���Ϊ ����
��ĸ��ţ�
A������Ũ�������ȹ��� B������Ũ�������½ᾧ������

��3���ữ��Cr2O72���ɱ�SO32-��ԭ��Cr3+�����ӷ���ʽΪ��
����CΪ ��Cr(OH)3���ܶȻ�����Ksp[Cr(OH)3]�� ��
��4������2CrO42����2H+
 Cr2O72����H2O���ͼʾװ�ã���Ϊ���Ե缫�����Na2CrO4��Һ��ȡNa2Cr2O7��ͼ���Ҳ�缫���ӵ�Դ�� ������缫��ӦʽΪ ��
Cr2O72����H2O���ͼʾװ�ã���Ϊ���Ե缫�����Na2CrO4��Һ��ȡNa2Cr2O7��ͼ���Ҳ�缫���ӵ�Դ�� ������缫��ӦʽΪ ��
��1���ٽ�Fe3+ˮ������Fe��OH��3����ȥ��Fe��OH��3
��2��A
��3��3SO32-+Cr2O72-+8H+=2Cr3++3SO42-+4H2O��H2SO4��1.0��10-32mol/L
��4������ ��4OH--4e-=O2+2H2O
��2��A
��3��3SO32-+Cr2O72-+8H+=2Cr3++3SO42-+4H2O��H2SO4��1.0��10-32mol/L
��4������ ��4OH--4e-=O2+2H2O
(1)�����ˮ�ⷴӦ�����ȷ�Ӧ���������ܴٽ�Fe3+ˮ������Fe��OH��3����ȥ��AΪFe��OH��3��
��2�������ܽ�����ߣ����Կ����¶Ƚϸ�ʱ�������¶ȵ����ߣ�Na2SO4�����٣����Բ�������Ũ�������ȹ��˵ķ���������A��ȷ��
��3���ữ��3SO32-+Cr2O72-+8H+=2Cr3++3SO42-+4H2O����Ϊ���ղ�Ʒ��Na2SO4��Ϊ����������ʣ���CΪ???�ǡ����ȫ����ʱ�������Ϊc ��1.0��10-5mol��L-1��Cr3+��ȫ����ʱpH=5��c��OH-��=1.0��10-9mol��L-1������Cr��OH��3��Ũ�Ȼ�����ΪKsp=1.0��10-5��1.0��10-9��3=1.0��10-32
��4������ʾ��ͼ֪��ͼ���Ҳ�Na2CrO4ת��ΪNa2Cr2O7����ҪH+��˵���Ҳ�缫����OH-�ŵ磬ʹH2O�ĵ���ƽ�������ƶ���H+���࣬�����Ҳ�缫�����ӵ�Դ���������缫����ʽΪ4OH--4e-=O2+2H2O
��2�������ܽ�����ߣ����Կ����¶Ƚϸ�ʱ�������¶ȵ����ߣ�Na2SO4�����٣����Բ�������Ũ�������ȹ��˵ķ���������A��ȷ��
��3���ữ��3SO32-+Cr2O72-+8H+=2Cr3++3SO42-+4H2O����Ϊ���ղ�Ʒ��Na2SO4��Ϊ����������ʣ���CΪ???�ǡ����ȫ����ʱ�������Ϊc ��1.0��10-5mol��L-1��Cr3+��ȫ����ʱpH=5��c��OH-��=1.0��10-9mol��L-1������Cr��OH��3��Ũ�Ȼ�����ΪKsp=1.0��10-5��1.0��10-9��3=1.0��10-32
��4������ʾ��ͼ֪��ͼ���Ҳ�Na2CrO4ת��ΪNa2Cr2O7����ҪH+��˵���Ҳ�缫����OH-�ŵ磬ʹH2O�ĵ���ƽ�������ƶ���H+���࣬�����Ҳ�缫�����ӵ�Դ���������缫����ʽΪ4OH--4e-=O2+2H2O

��ϰ��ϵ�д�
 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
�����Ŀ
 ��Ӧ2SO2��g��+O2��g�� 2SO3��g���ġ�H= kJ��mol-l��ʹ��V2O5��������÷�Ӧ�淴Ӧ�Ļ�� ������������䡱��С������
��Ӧ2SO2��g��+O2��g�� 2SO3��g���ġ�H= kJ��mol-l��ʹ��V2O5��������÷�Ӧ�淴Ӧ�Ļ�� ������������䡱��С������
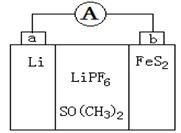
 LiNH2+2LiH����������Ϊ ���ѧʽ������270��ʱ���÷�Ӧ���������ų�H2���������﮿���Ϊ������ϣ������������ɴ�Li3N������ %����ȷ��0.1����
LiNH2+2LiH����������Ϊ ���ѧʽ������270��ʱ���÷�Ӧ���������ų�H2���������﮿���Ϊ������ϣ������������ɴ�Li3N������ %����ȷ��0.1���� FePO4֮���ת������طŵ�ʱ���������ķ�ӦΪLiXC6��Xe��
FePO4֮���ת������طŵ�ʱ���������ķ�ӦΪLiXC6��Xe�� XLi++6C��д����طŵ�ʱ�ĵ缫��Ӧ�Ļ�ѧ����ʽ ��
XLi++6C��д����طŵ�ʱ�ĵ缫��Ӧ�Ļ�ѧ����ʽ ��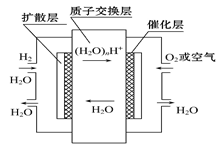

 (aq)
(aq) PbCO3(s)��SO42-(aq)
PbCO3(s)��SO42-(aq)
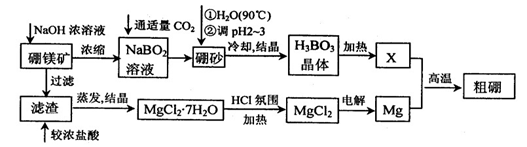
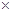 10-12������ҺpH=6ʱ (��С���û�С�)Mg(OH)2����������
10-12������ҺpH=6ʱ (��С���û�С�)Mg(OH)2����������